��Ŀ����
��14�֣���֪��NaAlO2�Ʊ�����Al(OH)3������ת����ϵ��ͼ��

��1����Ӧ�ڵ��Ȼ�ѧ����ʽΪ ��
��2��������������ת����ϵ���ݶ���˾�ҵ���һ�ּ�ݵĴ��������ȡAl2O3�ķ������������£�

������A�Ļ�ѧʽΪ ��
�ڲ����Ļ�ѧ��Ӧ����ʽΪ �����鲽����г����Ƿ�ϴ���ķ����� ��
�۲���������ȴ�ķ�����������Al(OH)3���ô�ʩ�������� ��
�ܹ�ҵ�Ͽɵ����������Al2O3�Ի��Al�������2.7kgAl��������������A�����ʵ�������Ϊ mol���������������Na[AlCl4]��NaCl�Ļ�������Al2O3���е����Al����������ӦΪ ��

��1����Ӧ�ڵ��Ȼ�ѧ����ʽΪ ��
��2��������������ת����ϵ���ݶ���˾�ҵ���һ�ּ�ݵĴ��������ȡAl2O3�ķ������������£�

������A�Ļ�ѧʽΪ ��
�ڲ����Ļ�ѧ��Ӧ����ʽΪ �����鲽����г����Ƿ�ϴ���ķ����� ��
�۲���������ȴ�ķ�����������Al(OH)3���ô�ʩ�������� ��
�ܹ�ҵ�Ͽɵ����������Al2O3�Ի��Al�������2.7kgAl��������������A�����ʵ�������Ϊ mol���������������Na[AlCl4]��NaCl�Ļ�������Al2O3���е����Al����������ӦΪ ��
��14�֣�
��1��NaAlO2(aq)+2H2O(l) = NaOH(aq)+����Al(OH)3��s�� ?H=��50kJ?mol?1��2�֣�
��2����NaOH��2�֣� ��2Al(OH)3 Al2O3+3H2O��2�֣�
Al2O3+3H2O��2�֣�
ȡ���һ��ϴ��Һ����ɫ��Ӧ���������û�л�ɫ���Ѿ�ϴ������֮��δϴ����2�֣�
��NaAlO2ˮ�����ɦ���Al(OH)3Ϊ���ȷ�Ӧ����ȴ���´�ʹNaAlO2ˮ�������ƶ����С����Al(OH)3��ǿ���е��ܽ⣨2�֣�
��100��2�֣� AlCl4-+3e?=Al+4Cl?��2�֣�
��1��NaAlO2(aq)+2H2O(l) = NaOH(aq)+����Al(OH)3��s�� ?H=��50kJ?mol?1��2�֣�
��2����NaOH��2�֣� ��2Al(OH)3
 Al2O3+3H2O��2�֣�
Al2O3+3H2O��2�֣�ȡ���һ��ϴ��Һ����ɫ��Ӧ���������û�л�ɫ���Ѿ�ϴ������֮��δϴ����2�֣�
��NaAlO2ˮ�����ɦ���Al(OH)3Ϊ���ȷ�Ӧ����ȴ���´�ʹNaAlO2ˮ�������ƶ����С����Al(OH)3��ǿ���е��ܽ⣨2�֣�
��100��2�֣� AlCl4-+3e?=Al+4Cl?��2�֣�
�����������1������д����Ӧ�ڵĻ�ѧ����ʽ��ע��״̬NaAlO2(aq)+2H2O(l) = NaOH(aq)+����Al(OH)3��s�����ݸ�˹��������÷�Ӧ���ʱ�?H=��1/2?H1 + 1/2?H3=��50kJ?mol?1���ɵ��Ȼ�ѧ����ʽΪ��
��2����Al2O3Ϊ�����������������AΪNaOH��
�ڲ����ΪAl(OH)3���շֽ�����Al2O3�����Ի�ѧ����ʽΪ��2Al(OH)3
 Al2O3+3H2O��������г������û��ϴ�Ӹɾ�������Ậ��Na+�����Լ��鷽��Ϊ��ȡ���һ��ϴ��Һ����ɫ��Ӧ���������û�л�ɫ���Ѿ�ϴ������֮��δϴ����
Al2O3+3H2O��������г������û��ϴ�Ӹɾ�������Ậ��Na+�����Լ��鷽��Ϊ��ȡ���һ��ϴ��Һ����ɫ��Ӧ���������û�л�ɫ���Ѿ�ϴ������֮��δϴ�����۸��ݣ�1�����Ȼ�ѧ����ʽ��֪NaAlO2ˮ�����ɦ���Al(OH)3Ϊ���ȷ�Ӧ����ȴ���´�ʹNaAlO2ˮ�������ƶ����С����Al(OH)3��ǿ���е��ܽ⡣
�ܸ��ݻ�ѧ����ʽ�ɵö�Ӧ��ϵ��Al �� NaOH ����n��NaOH��=2700g��27g/mol=100mol�������Ϸ���AlCl4-�õ��ӷ�Ӧ���缫����ʽΪ��AlCl4-+3e?=Al+4Cl?

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
�����Ŀ
 HCOOCH3(g)+2H2(g) ��H>0
HCOOCH3(g)+2H2(g) ��H>0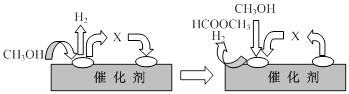


 CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±���
CO2(g)+H2(g)�������ʵ�����CO(g)��H2O(g)�����ܱ������з�Ӧ��ƽ��ʱ��ý�����±��� 2Fe(s)+3CO2(g);��H="-24.8" kJ/mol
2Fe(s)+3CO2(g);��H="-24.8" kJ/mol CO(g)
CO(g) Fe3O4(s)+
Fe3O4(s)+