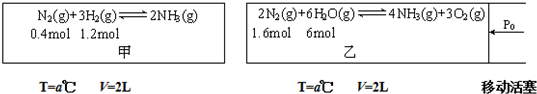��Ŀ����
1����֪M��Q��X��Y��Z��ǰ��������Ԫ�أ�������ϡ������Ԫ�أ����й����ʺ������Ϣ���±���| Ԫ�� | �����Ϣ |
| M | ����������Ӧ��ˮ�����ܰ�1��1�������������ȵ����������� |
| Q | ��������������������������֮��Ϊ4���ǽ�������ͬ����Ԫ������ǿ |
| X | �䵥��Ϊ����ɫ���� |
| Y | ij�ֺ���ԭ�ӵ�������Ϊ56��������Ϊ30 |
| Z | XԪ�صĵ�����ZԪ�صĵ�����ȼ�գ���������������ɫ���� |
��3��M��Q��Z�����ӵİ뾶�ɴ�С��˳��ΪCl-��O2-��Na+�������ӷ��ű�ʾ��
��4����һ����ѧ����ʽ��ʾ֤���ǽ�����Q��X��H2S+Cl2=2HCl+S��
��5��5.6L��״���µ�X�ļ��⻯����������Z��������ȫȼ������Һ̬ˮʱ���ų�������Ϊ356.8kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ2H2S��g��+3O2��g��=2SO2��g��+2H2O��l����H=-2854.4kJ/mol
��6���ڼ��������£�Q�ĵ��ʿ���YZ2-��Ӧ�Ʊ�һ�ֿ����ھ�ˮ����YZ42-���÷�Ӧ�����ӷ���ʽΪ3Cl2+2FeO2-+8OH-=2FeO42-+6Cl-+4H2O��
���� M��Q��X��Y��Z��ǰ��������Ԫ�أ�������ϡ������Ԫ�أ���M����������Ӧ��ˮ�����ܰ�1��1�������������ȵ����������ӣ���MΪNa��X����Ϊ����ɫ���壬��XΪSԪ�أ�Q��������������������������֮��Ϊ4���ǽ�������ͬ����Ԫ�������QΪClԪ�أ�Y��ij�ֺ���ԭ�ӵ�������Ϊ56��������Ϊ30����������Ϊ56-30=26����YΪFe��XԪ�صĵ�����ZԪ�صĵ�����ȼ�գ���������������ɫ���棬��ZΪOԪ�أ��ݴ˽��
��� �⣺M��Q��X��Y��Z��ǰ��������Ԫ�أ�������ϡ������Ԫ�أ���M����������Ӧ��ˮ�����ܰ�1��1�������������ȵ����������ӣ���MΪNa��X����Ϊ����ɫ���壬��XΪSԪ�أ�Q��������������������������֮��Ϊ4���ǽ�������ͬ����Ԫ�������QΪClԪ�أ�Y��ij�ֺ���ԭ�ӵ�������Ϊ56��������Ϊ30����������Ϊ56-30=26����YΪFe��XԪ�صĵ�����ZԪ�صĵ�����ȼ�գ���������������ɫ���棬��ZΪOԪ�أ�
��1����һ���ں���2��Ԫ�أ��ֱ��ڵ�1�С���18�У��ڶ�����������3-10��û��Ԫ�أ�Fe���ڵ������ڡ���8�У���ͼ��ʾ��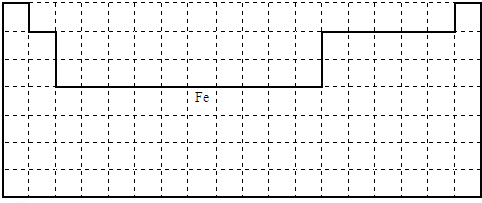 ��
��
�ʴ�Ϊ�� ��
��
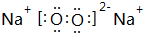
��2��M��Z�γɵĺ��й��ۼ��Ļ�����ΪNa2O2������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵ��Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��Cl-��O2-��Na+��
�ʴ�Ϊ��Cl-��O2-��Na+��
��4�����õ���֮����û��ȿ���֤���ǽ�����ǿ������ʾ֤���ǽ�����Cl��S��Ӧ����ʽΪ��H2S+Cl2=2HCl+S����
�ʴ�Ϊ��H2S+Cl2=2HCl+S����
��5��X�ļ��⻯��ΪH2S�����ʵ���Ϊ$\frac{5.6L}{22.4L/mol}$=0.25mol��������������������ȫȼ������Һ̬ˮʱ���ų�������Ϊ356.8kJ��2mol����ȼ�շų�������Ϊ356.8kJ��$\frac{2mol}{0.25mol}$=2854.4kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��2H2S��g��+3O2��g��=2SO2��g��+2H2O��l����H=-2854.4kJ/mol��
�ʴ�Ϊ��2H2S��g��+3O2��g��=2SO2��g��+2H2O��l����H=-2854.4kJ/mol��
��6���ڼ���������Cl2����FeO2-��Ӧ�Ʊ�һ�ֿ����ھ�ˮ����FeO42-����������ԭΪ���ӣ��÷�Ӧ�����ӷ���ʽΪ��3Cl2+2FeO2-+8OH-=2FeO42-+6Cl-+4H2O��
�ʴ�Ϊ��3Cl2+2FeO2-+8OH-=2FeO42-+6Cl-+4H2O��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��漰Ԫ�����ڱ���Ԫ�������ɡ����뾶�Ƚϡ��Ȼ�ѧ����ʽ��������ԭ��Ӧ�ȣ��ؼ����ƶ�Ԫ�أ����ؿ���ѧ���Ի���֪ʶ��Ǩ�����ã��Ѷ��еȣ�

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�| A�� | ̼������ͭ��ϸ��¼��� | B�� | �������Һ���Ȼ�þ��Һ | ||
| C�� | ��������Һ����������Һ | D�� | ����������Һ����������Һ |
��Na2CO3-�Ʋ����� ��SiO2-̫���ܵ�أ� ��Na2SiO3-ľ�ķ������ ��NH3-�������
��Al2O3-���Ӹֹ죻 ��NaClO-��������Ư��֯�� ��Fe2O3-��ɫ�����Ϳ�ϣ�
| A�� | 4�� | B�� | 5�� | C�� | 6�� | D�� | 7�� |
��1�����̼�Ȼ�ԭ-�Ȼ�����ʵ���������Ʊ�������������ص��Ȼ�ѧ����ʽ���£�
2Al2O3��s��+2AlCl3��g��+6C��s���T6AlCl��g��+6CO��g����H=akJ•mol-1
3AlCl��g���T2Al��l��+AlCl3��g����H=bkJ•mol-1
��ӦAl2O3��s��+3C��s���T2Al��l��+3CO��g���ġ�H=��0.5a+b��kJ•mol-1���ú�a��b�Ĵ���ʽ��ʾ����
��2���û���̿��ԭ�����Դ����������ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO��������ӦC��s��+2NO��g��?N2��g��+CO2��g����H=QkJ•mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| ʱ�䣨min�� Ũ�ȣ�mol•L-1�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
| N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
| CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������ad������ĸ��ţ���
a��ͨ��һ������NO
b������һ�����Ļ���̿
c��������ʵĴ���
d���ʵ���С���������
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ3��1��1����Q��0���������������
���ھ��Ⱥ��������£����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬��������bcd����ѡ���ţ���
a����λʱ��������2nmol NO��g����ͬʱ����nmol CO2��g��
b����Ӧ��ϵ���¶Ȳ��ٷ����ı�
c�����������ܶȲ��ٷ����ı�
d����Ӧ��ϵ��ѹǿ���ٷ����ı䣮
| A�� | ���� | B�� | θ����� | C�� | ��Ĥ�� | D�� | ���˸�Ⱦ |