��Ŀ����
����Ŀ��I .�ϳɰ���ҵ���ִ�ũҵ��������ҵ�У�������Ҫ�ĵ�λ
��֪��N2 (g) +3H2 (g) ![]() 2NH3 (g) ��H=-92 kJ��mol-1
2NH3 (g) ��H=-92 kJ��mol-1
(1)�����йظ÷�Ӧ���ʵ���������ȷ����(ѡ�����)____________��
a.�����¶ȿ����������Ӱٷ������ӿ췴Ӧ����
b.����ѹǿ�����������Ӱٷ����������Լӿ췴Ӧ����
c.ʹ�ô�������ʹ��Ӧ�����ƽ���������ߣ��ӿ췴Ӧ����
d.����������������£����������ı������С���Է�Ӧ����������Ӱ��
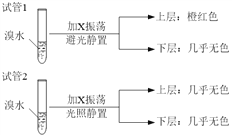
(2)���ںϳɰ���Ӧ���ԣ���ͼ�й�ͼ��һ����ȷ����(ѡ�����) ______________��

II.��2L���ܱ������г���7.6molNO��3.8mol O2���������·�Ӧ��
��2NO(g) + O2(g) =2NO2 (g) ��2NO2 (g) ==N2O4 (g)
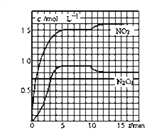
���NO2��N2O4��Ũ�ȱ仯��ͼ��ʾ��0~10minά�������¶�ΪT1�棬10min�����߲�ά���������¶�ΪT2�档
(1)����ǰ5min N2O4��Ӧ��ƽ������Ϊ________________________��
(2)����T1��ʱ��Ӧ���Ļ�ѧƽ�ⳣ��Ϊ_______________________��
(3)����ʼʱ��������г���3.6mol NO2��2.0mol N2O4���ж�T1��ʱ��Ӧ�����еķ���_____(���������ƶ�)��������ﵽƽ��ʱN2O4�����ʵ���Ϊ_____mol��
���𰸡� abd ac 0.18mol/ (L��min) 0.4 ���� 2.2mol
��������I����1��a�������¶ȿ����������Ӱٷ�������Ч��ײ�Ĵ������ӣ���ӿ췴Ӧ���ʣ���a��ȷ��b������ѹǿ��������λ����������Ŀ����ӿ췴Ӧ���ʣ���b��ȷ��c��ʹ�ô��������ͷ�Ӧ�Ļ�ܣ��ӿ췴Ӧ���ʣ�����Ӧ�����ƽ���������䣬��c����d���������������������Ӧ���ʣ���d��ȷ���ʴ�Ϊabd��
��2��a�������¶ȣ�ƽ�������ƶ������������������С������Ӧ����ƽ���ʱ�����̣�����ȷ��b����Ӧ����ƽ��ʱ��������Ũ�ȱ仯��֮�ȵ����������֮�ȣ������백��Ũ�ȱ仯��֮��Ϊ3��2���ʴ���c��ʹ�ô����ӿ췴Ӧ���ʣ����̷�Ӧʱ�䣬����Ӱ��ƽ���ƶ����������⣬����ȷ���ʴ�Ϊac��
������1����ͼ��֪5minʱN2O4Ũ��Ϊ0.9mol/L����ǰ5min��Ӧ��ƽ������v��N2O4��=![]() =0.18mol/��Lmin����
=0.18mol/��Lmin����
��2��T1��ʱ��Ӧ��2NO2��g��N2O4��g������5min�ﵽƽ�⣬��ʱN2O4Ũ��Ϊ0.9mol/L��NO2Ũ��Ϊ1.5mol/L����ѧƽ�ⳣ��K=![]() =
=![]() =0.4��
=0.4��
��3����ʼʱ��������г���3.6molNO2��2.0molN2O4����ʱQc=![]() =0.31��K����Ӧ������У�
=0.31��K����Ӧ������У�
��ת������������Ϊx��������ʽ��
2NO2��g��N2O4��g��
cʼ��1.81.0
cת��2xx
cƽ��1.8-2x1.0+x
��ͬ�¶��£�K���䣬��:![]() �T0.4����x=0.1����ƽ��ʱN2O4�����ʵ���Ϊ��1+0.1��mol/L��2L=2.2mol��
�T0.4����x=0.1����ƽ��ʱN2O4�����ʵ���Ϊ��1+0.1��mol/L��2L=2.2mol��

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�