��Ŀ����
�о� ��
�� ��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��1�� ��ʹ
��ʹ �������������ڶ����ⶨCO�ĺ�������֪��
�������������ڶ����ⶨCO�ĺ�������֪��

д��CO��g���� ��Ӧ����
��Ӧ���� ���Ȼ�ѧ����ʽ��________________��
���Ȼ�ѧ����ʽ��________________��
��2��CO������ȼ�ϵ�أ���KOH��Һ������ʣ��������ֱ����CO�Ϳ��������������У�K+����_______��(���������)��������Ӧ����ʽΪ��___________________��
��3�����Ͱ��������������Ļ�ѧԭ���Dz��ð�ˮ���������е�SO2������һ��������
�����������ղ��ﷴӦ���ü������ŵ�����ܻ�������SO2�⣬���ܵõ�һ�ָ��Ϸ��ϡ�
�ٸø��Ϸ��Ͽ��ܵĻ�ѧʽΪ___________(д��һ�ּ���)��
������ˮ�� ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��
ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��
������������ʵĵ���ƽ�ⳣ�����£���ˮ

���������Һ��ͨ��________�����ʹ��Һ�����ԡ�(�SO2����NH3��)
��ʱ��Һ�� ________2�������������������
________2�������������������
��4�� ����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�
����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ� ��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
 ��
�� ��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣��1��
 ��ʹ
��ʹ �������������ڶ����ⶨCO�ĺ�������֪��
�������������ڶ����ⶨCO�ĺ�������֪��
�CO��g����
 ��Ӧ����
��Ӧ���� ���Ȼ�ѧ����ʽ��________________��
���Ȼ�ѧ����ʽ��________________����2��CO������ȼ�ϵ�أ���KOH��Һ������ʣ��������ֱ����CO�Ϳ��������������У�K+����_______��(���������)��������Ӧ����ʽΪ��___________________��
��3�����Ͱ��������������Ļ�ѧԭ���Dz��ð�ˮ���������е�SO2������һ��������
�����������ղ��ﷴӦ���ü������ŵ�����ܻ�������SO2�⣬���ܵõ�һ�ָ��Ϸ��ϡ�
�ٸø��Ϸ��Ͽ��ܵĻ�ѧʽΪ___________(д��һ�ּ���)��
������ˮ��
 ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��
ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��������������ʵĵ���ƽ�ⳣ�����£���ˮ


���������Һ��ͨ��________�����ʹ��Һ�����ԡ�(�SO2����NH3��)
��ʱ��Һ��
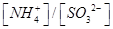 ________2�������������������
________2���������������������4��
 ����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�
����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ� ��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________����ÿ��2�֣�
��1��5CO(g) + I2O5(s) = 5CO2 + I2(s) ?H= -1377.22kJ?mol?1
��2������O2 + 2H2O +4e? = 4OH?
��3���� (NH4)3PO4��(NH4)2HPO4��NH4H2PO4
�ڼ� �� SO2 >
��4��3Fe2+ + NO3? + 4H+ =3Fe3+ +NO��+ 2H2O
��1��5CO(g) + I2O5(s) = 5CO2 + I2(s) ?H= -1377.22kJ?mol?1
��2������O2 + 2H2O +4e? = 4OH?
��3���� (NH4)3PO4��(NH4)2HPO4��NH4H2PO4
�ڼ� �� SO2 >
��4��3Fe2+ + NO3? + 4H+ =3Fe3+ +NO��+ 2H2O
�����������1����д����ѧ����ʽ�����������ʵ�״̬��Ȼ����ݸ�˹��������?H��
?H= ��
 ?H1 +
?H1 + 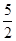 ?H2������������ݿɵô𰸡�
?H2������������ݿɵô𰸡���2��ԭ��ص������Һ�У����������������������ΪKOH��Һ������������ӦΪO2��H2O���������µõ�������OH?��
��3���ٷ�����Ӧ���������������Ӧ���������ᷴӦ�������������ͬ������(NH4)3PO4��(NH4)2HPO4��NH4H2PO4��
�ڰ�ˮ��SO2ǡ����ȫ��Ӧ����(NH4)2SO3��ˮ���Լ��ԡ�
����Ϊ������Һ�Լ��ԣ�����ͨ��SO2���к�OH?��ʹ��Һ�����ԣ����ݵ���غ��֪��[NH4+]+[H+]=[OH?]+2[SO32?]+[HSO3?]����Һ����[H+]=[OH?],��[NH4+]=2[SO32?]+[HSO3?]������[NH4+]/[SO32?]>2��
��4��������Ϣ�ҳ���Ӧ����������ƽ�ɵ����ӷ���ʽ��

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ
 2SO3(g)��ƽ�ⳣ��Ϊ ��
2SO3(g)��ƽ�ⳣ��Ϊ ��




 ����
���� H[CuCl2]����Ӧ��������Һ��������ˮ����CuCl���ɡ�ʵ������м���Ũ�����Ŀ���� ����c(Cl-)=2��10-3 mol��L��1ʱ, c(Cu+-)= mol��L��1����֪��Ksp(CuCl)=1.7��10-7
H[CuCl2]����Ӧ��������Һ��������ˮ����CuCl���ɡ�ʵ������м���Ũ�����Ŀ���� ����c(Cl-)=2��10-3 mol��L��1ʱ, c(Cu+-)= mol��L��1����֪��Ksp(CuCl)=1.7��10-7