��Ŀ����
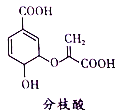
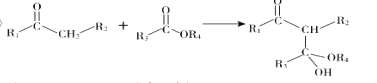
����Ŀ��ɫͪ�����K���п�������Ѫ֬���������ԣ���ϳ�·�����£�
��֪��
��
��
![]()
��
��![]() ����������
����������
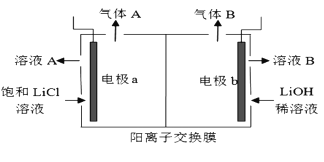
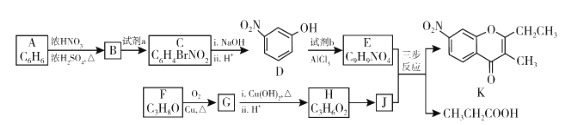
��1��A�Ľṹ��ʽ��_________������ϵͳ��������F��������__________��
��2��B��C�����Լ�a��__________���Լ�b�Ľṹ��ʽ��_________��
��3��C��������![]() ��Ӧ�Ļ�ѧ����ʽΪ__________��
��Ӧ�Ļ�ѧ����ʽΪ__________��
��4��G������![]() ��Ӧ�Ļ�ѧ����ʽΪ__________��
��Ӧ�Ļ�ѧ����ʽΪ__________��
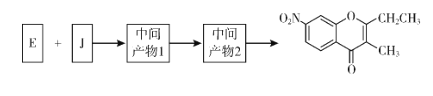
��5����֪����![]() ����J�����������ֻ�ѧ������ͬ������E��JΪԭ�Ϻϳ�K��Ϊ������Ӧ��д���йػ�����Ľṹ��ʽ��__________
����J�����������ֻ�ѧ������ͬ������E��JΪԭ�Ϻϳ�K��Ϊ������Ӧ��д���йػ�����Ľṹ��ʽ��__________
���𰸡� 1-����
1-���� ![]() ��
��![]() ����
����![]() ��
�� 

![]()

![]()
![]() E:
E:  ��J:
��J: ![]()
����1��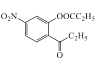 ������2��
������2��
��������
�ɷ���ʽ��֪AΪ����ת����ϵ��֪BΪ![]() ����D��֪CΪ
����D��֪CΪ �����E�ķ���ʽ�Լ�K�Ľṹ��ʽ��֪EΪ
�����E�ķ���ʽ�Լ�K�Ľṹ��ʽ��֪EΪ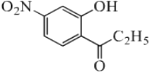 ���Լ�bΪ
���Լ�bΪ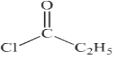 ��FΪCH3CH2CH2OH��GΪCH3CH2CHO��HΪCH3CH2COOH��JΪ
��FΪCH3CH2CH2OH��GΪCH3CH2CHO��HΪCH3CH2COOH��JΪ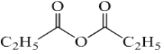 ���Դ˽����⡣
���Դ˽����⡣
(1)�����Ϸ�����֪AΪ![]() ��FΪCH3CH2CH2OH��Ϊ1-������
��FΪCH3CH2CH2OH��Ϊ1-������
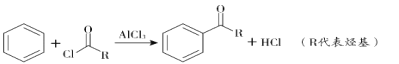
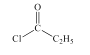
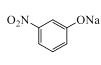
(2)B���巢��ȡ����Ӧ����ҪFeBr3�������������Լ�a��Br2��FeBr3���Լ�bΪ ��
��
(3)CΪ ��������������Һ��Ӧ�Ļ�ѧ����ʽΪ
��������������Һ��Ӧ�Ļ�ѧ����ʽΪ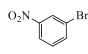
![]()
![]() ��
��
(4)G������Cu(OH)2��Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
(5)EΪ ��JΪ
��JΪ ���������֪�����ȷ�Ӧ�����м����1�Ľṹ��ʽΪ
���������֪�����ȷ�Ӧ�����м����1�Ľṹ��ʽΪ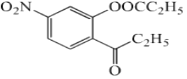 ��Ȼ�������м����2�Ľṹ��ʽΪ
��Ȼ�������м����2�Ľṹ��ʽΪ ���������ȥ��Ӧ������K��
���������ȥ��Ӧ������K��

 �Ķ��쳵ϵ�д�
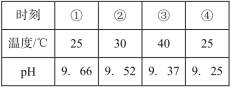
�Ķ��쳵ϵ�д�����Ŀ�������ᡢ���ᡢ��������ᶼ��ǿ�ᣬ��������ˮ��Һ�в��������ij�¶������������ڱ������еĵ��볣�����±�����ӱ������ж�����˵������ȷ���ǣ� ��
�� | HClO4 | H2SO4 | HCl | HNO3 |
Ka | 1.6��10��5 | 6.3��10��9 | 1.6��10��9 | 4.2��10��10 |
A.�ڱ��������������ᶼû����ȫ����
B.�ڱ������и�������������������ǿ����
C.�ڱ�����������ĵ��뷽��ʽΪH2SO4=2H����SO42-
D.ˮ�������������ǿ��û����������������������������������ǿ��