��Ŀ����
3��һˮ�������İ���ͭ����[Cu��NH3��4]SO4��•H2OΪ����ɫ���壬�ڹ�ҵ����;�㷺��������ɱ�����ýȾ����ijѧϰС��̽���Ʊ��þ��岢�ⶨ����ɵ�ʵ��������£���������Ʊ�ԭ�������̣�
CuSO4+4NH3+H2O�T[Cu��NH3��4]SO4��•H2O
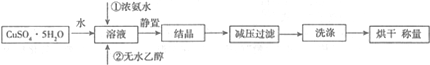
�ش��������⣺
��1����ˮ�Ҵ������ý������ܽ��ԣ������ھ������������ɾ�����¶�����60���ԭ�����¶ȹ������Ⱦ����ֽ⣻
��2��ʵ���Ƶò��ᄃ����������Cu2��OH��2SO4���ʣ��������������ʵ�ԭ�������ͭ���ӷ���ˮ�⣻
���������ܽ���������Һ�У�����NaI��Һ��Cu2+����I���ò���I2�Ͱ�ɫ�������÷�Ӧ�����ӷ���ʽΪ2Cu2++4I-�T2CuI��+I2������Na2S2O3��Һ�ζ�I2�����ɵ�Cu2+������
�������е������IJⶨ
ʵ��װ����ͼ��ʾ����ȡ0.9380g[Cu��NH3��x]SO4•H2O����[M=��178+17x��g/mol]����ƿa�У�ͨ����Һ©������ƿa�еμ�10%NaOH��Һ����1.000mol/L��NaOH���ζ�b��ʣ���HCl��

��4���ζ��ܶ�����ͼ��ʾ�����ı�Һ16.00mL��[Cu��NH3��x]SO4•H2O��x=3.56���Ʋ����ֵ��x��������ֵƫС��ԭ���Ǽ����NaOH��Һ���㣬û�м��ȣ������ɵİ���û����ȫ�ݳ�����
������SO42-�����IJⶨ--������
��5����ȡһ����������������ϡ�����ܽ⣬��BaCl2������Һ�У����õõ�BaSO4�����������������ȫ�IJ���Ϊ���ã����ϲ���Һ�м�������BaCl2��Һ�����������ɣ���˵��������ȫ��
���� ��1��[Cu��NH3��4]SO4��•H2O����������ˮ���������Ҵ������Ⱦ����ֽ⣻
��2��ʵ���Ƶò��ᄃ����������Cu2��OH��2SO4���ʣ��������������ʵ�ԭ�����ͭ���ӷ���ˮ�⣻
����ͭ���ӱ�I-��ԭΪ��ͭ���ӣ������ӱ�����ΪI2������ԭ���غ�͵�ʧ�����غ���д��
��4�����ݵζ��ܶ����ÿ�ʼ-�����������ı�Һ�����Cu��NH3��xSO4•H2O���������Ʒ�Ӧ���ɵİ������������գ������������Ƶζ�ʣ������ᣬ�������ĵ�������������백����Ӧ�����ᣬ�Լ����������ʵ��������ݾ����백�������ʵ����Ĺ�ϵ���x�������ɵİ���ƫ�ٻ���û����ȫ�ݳ������������ʵ���ƫС��xֵƫС��
��5��SO42-���Ӻ�Ba2+���ӷ�Ӧ�����ɰ�ɫ����������İ�ɫ������
��� �⣺��1������ˮ�Ҵ��ܽ⣬�������ܽ��ԣ������ھ�����������¶ȹ����ȹ����о������ȷֽ⣬������ˮ�Ҵ������ý������ܽ��ԣ������ھ������������ɾ�����¶�Ϊ60���ԭ�����¶ȹ������Ⱦ����ֽ⣬�ʴ�Ϊ���������ܽ��ԣ������ھ�����������¶ȹ������Ⱦ����ֽ⣻
��2��ʵ���Ƶò��ᄃ����������Cu2��OH��2SO4���ʣ�˵�������������ӱ���ϣ�������Ϊͭ���ӷ���ˮ�⣬�ʴ�Ϊ��ͭ���ӷ���ˮ�⣻
����ͭ���ӱ�I-��ԭΪ��ͭ���ӣ������ӱ�����ΪI2�������ӷ���ʽΪ��2Cu2++4I-�T2CuI��+I2���ʴ�Ϊ��2Cu2++4I-�T2CuI��+I2����
��4���ɵζ��ܶ�����ͼ��ʾ���ζ��ܶ�����ʼΪ0.10ml������Ϊ16.10���������ı�Һ���Ϊ16.10-0.10=16.00ml���ζ����ĵ���������Ϊn��NaOH��=cV=1.000mol/L��0.01600L=0.01600mol�������������Ʒ�Ӧ������Ϊ0.01600mol���백����Ӧ������Ϊ��1.000mol/L��0.0300L-0.01600mol=0.014000mol�������������ʵ���Ϊ0.014000mol��
�ɣ�NH3��xSO4•H2O��xNH3��
1 x
$\frac{0.9380}{178+17x}$mol 0.014000mol
$\frac{0.9380}{178+17x}$=$\frac{0.014}{x}$
��ã�x=3.56��
�������NaOH��Һ���㣬Cu��NH3��xSO4û����ȫ��Ӧ�����ɵİ���ƫ�٣���û�м��������ɰ������ܴ���Һ�лӷ���������ʹ���������յİ���ƫ�٣�����û����ȫ�ݳ������������ʵ���ƫС����xֵƫС��
�ʴ�Ϊ��16.00��3.56�������NaOH��Һ���㣬û�м��ȣ������ɵİ���û����ȫ�ݳ�����
��5������SO42-���Ӻ�Ba2+���ӷ�Ӧ�����ɰ�ɫ����������İ�ɫ���������飬��������Ϊ�����ã����ϲ���Һ�м�������BaCl2��Һ�����������ɣ���˵��������ȫ��
�ʴ�Ϊ�����ã����ϲ���Һ�м�������BaCl2��Һ�����������ɣ���˵��������ȫ��
���� ���⿼�������ʵ��Ʊ�ʵ�鷽������ơ�������ɵIJⶨ����Ŀ�Ѷ��еȣ������ۺ��Խ�ǿ��֪ʶ���ȫ�棬��ȷ�����Ʊ�ԭ��Ϊ���ؼ��������ֿ�����ѧ���ķ���������������������������ѧʵ��������

| A�� | ά����C�ܽ�+5����������As2O3 | |
| B�� | ά����C���л�ԭ�� | |
| C�� | ����к���ά����C | |
| D�� | ������˪�Ĺ�������Ԫ�ط���������Ӧ |

����˵���в���ȷ���ǣ�������
| A�� | �ù�����һ���µ������������� | |
| B�� | C2H5OSO3H�������� | |
| C�� | �ù����Т١����Ǽӳɷ�Ӧ | |
| D�� | �ù����Тۡ��ܿɿ��������ˮ�ⷴӦ |
| A�� | ������ ����� | B�� | ������ ���� | C�� | ��Һ ��Һ | D�� | ��Һ ��ɢϵ |
 ����ϩ�����ܵĽṹ�У�������
����ϩ�����ܵĽṹ�У�������| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | ����ƿ���������ɫ���� | B�� | ����ƿ���л��dz��� | ||
| C�� | ����ƿ�ڱ�������״Һ����� | D�� | ����ƿ�����ػ�ɫ�̳��� |

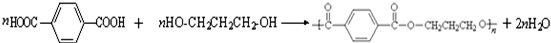 ��
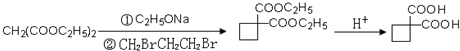
�� ��������Ƴ������ķ�Ӧ����ͼ��
��������Ƴ������ķ�Ӧ����ͼ��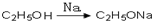 ��
��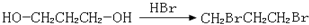 ��
�� ��
��