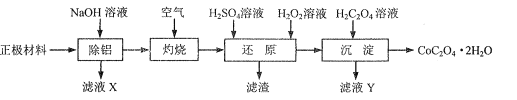
��Ŀ����
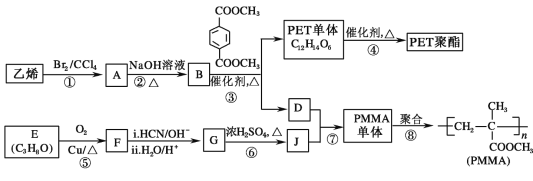
����Ŀ���߷��Ӳ��� PET ������֬�� PMMA �ĺϳ�·�����£�
��֪����.RCOOR����R��18OH RCO18OR����R��OH(R��R����R����������)
RCO18OR����R��OH(R��R����R����������)
II. 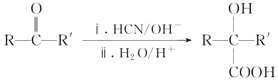 (R��R����������)
(R��R����������)
(1)�ٵķ�Ӧ������_____________��
(2)�ڵĻ�ѧ����ʽΪ____________��
(3)PMMA ���������������_____��_____��
(4)F �ĺ˴Ź���������ʾֻ��һ��壬�ݵĻ�ѧ����ʽΪ��_________��
(5)G �Ľṹ��ʽΪ______________��
(6)����˵����ȷ����__________(����ĸ���)��
a����Ϊ������Ӧ
b��B �� D ��Ϊͬϵ��
c��D �ķе��̼ͬԭ������������
d��1mol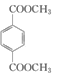 ������ NaOH ��Һ��Ӧʱ��������� 4 mol NaOH
������ NaOH ��Һ��Ӧʱ��������� 4 mol NaOH
(7)J ��ij��ͬ���칹���� J ������ͬ�����ţ���Ϊ˳ʽ�ṹ����ṹ��ʽ�ǣ�______��
(8)д���� PET �����Ʊ� PET ���������� B �Ļ�ѧ����ʽ��__________��
(9)д���ߵĻ�ѧ����ʽΪ��_______________��
���𰸡��ӳɷ�Ӧ BrCH2CH2Br+2NaOH![]() HOCH2CH2OH+2NaBr ̼̼˫�� ���� 2
HOCH2CH2OH+2NaBr ̼̼˫�� ���� 2![]() +O2
+O2 ![]() 2
2![]() +2H2O
+2H2O 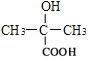 ac
ac ![]() n
n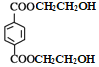
![]()
![]() +(n-1)HOCH2CH2OH
+(n-1)HOCH2CH2OH 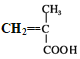 + CH3OH
+ CH3OH![]()
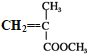 +H2O
+H2O
��������
����ͼ�У���ϩ��Br2/CCl4�����ӳɷ�Ӧ����BrCH2CH2Br(A)��A��NaOH��Һ�з���ˮ������HOCH2CH2OH(B)��NaBr��B��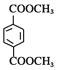 ������������Ӧ������
������������Ӧ������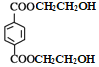 (PET����)��CH3OH(D)��PET������������
(PET����)��CH3OH(D)��PET������������![]() ��HOCH2CH2OH(B)��PMMA�Ľṹ��ʽΪ
��HOCH2CH2OH(B)��PMMA�Ľṹ��ʽΪ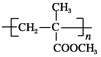 �����Ƶ����ĵ���Ϊ
�����Ƶ����ĵ���Ϊ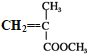 ����JΪ
����JΪ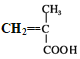 ����GΪ
����GΪ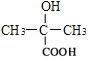 ��FΪ
��FΪ![]() ��EΪ
��EΪ![]() ��
��
(1)�����Ϸ���֪���ٵķ�Ӧ�����Ǽӳɷ�Ӧ����Ϊ���ӳɷ�Ӧ��
(2)���У�BrCH2CH2Br(A)��NaOH��Һ�з���ˮ������HOCH2CH2OH(B)��NaBr����ѧ����ʽΪBrCH2CH2Br+2NaOH![]() HOCH2CH2OH+2NaBr����Ϊ��BrCH2CH2Br+2NaOH
HOCH2CH2OH+2NaBr������BrCH2CH2Br+2NaOH![]() HOCH2CH2OH+2NaBr��
HOCH2CH2OH+2NaBr��
(3)PMMA����Ϊ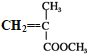 ��������������̼̼˫������������Ϊ��̼̼˫����������
��������������̼̼˫������������Ϊ��̼̼˫����������
(4)F �ĺ˴Ź���������ʾֻ��һ��壬�ݵĻ�ѧ����ʽΪ��2![]() +O2
+O2 ![]() 2
2![]() +2H2O������2
+2H2O������2![]() +O2
+O2 ![]() 2
2![]() +2H2O��
+2H2O��
(5)�����Ϸ���֪��G �Ľṹ��ʽΪ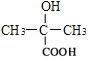 ����Ϊ��
������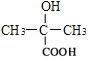 ��
��
(6)a����Ϊ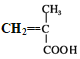 ��CH3OH��Ӧ����
��CH3OH��Ӧ����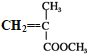 ��H2O����Ӧ����Ϊ������Ӧ��a��ȷ��
��H2O����Ӧ����Ϊ������Ӧ��a��ȷ��
b��HOCH2CH2OH(B)��CH3OH(D)��������Ŀ��ͬ�����߲���Ϊͬϵ�b����ȷ��
c��CH3OH(D)�ķе��̼ͬԭ����������(CH4)�ߣ���ΪCH3OH���γɷ��Ӽ�������c��ȷ��
d��1mol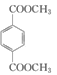 ������ NaOH ��Һ��Ӧʱ��������� 2mol NaOH��d����ȷ��
������ NaOH ��Һ��Ӧʱ��������� 2mol NaOH��d����ȷ��
��ѡac����Ϊ��ac��
(7)J Ϊ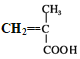 ����ij��ͬ���칹���� J ������ͬ�����ţ���Ϊ˳ʽ�ṹ����ṹ��ʽ�ǣ�
����ij��ͬ���칹���� J ������ͬ�����ţ���Ϊ˳ʽ�ṹ����ṹ��ʽ�ǣ�![]() ����Ϊ��
������![]() ��
��
(8)�� PET �����Ʊ� PET ���������� B �Ļ�ѧ����ʽ��n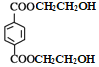
![]()
![]() +(n-1)HOCH2CH2OH������n
+(n-1)HOCH2CH2OH������n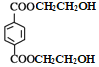
![]()
![]() +(n-1)HOCH2CH2OH��
+(n-1)HOCH2CH2OH��
(9)�ߵĻ�ѧ����ʽΪ��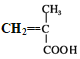 + CH3OH
+ CH3OH![]()
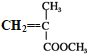 +H2O������
+H2O������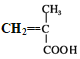 + CH3OH
+ CH3OH![]()
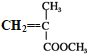 +H2O��
+H2O��