��Ŀ����
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2���� ��
��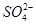 ��Cl����I����
��Cl����I����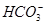 ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺
�ɴ��жϣ�
(1)��Һ��һ�����ڵ������� ����Һ�п϶������ڵ������� ��
(2)Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ�鼰��Ӧ���������ӵ�����(��Ϊ��Һ��Ӧ��˵��ʹ���Լ������ƣ�����д��ϸ����) ��
(3)д��ʵ��������з�Ӧ�����ӷ���ʽ�� ��
 ��
��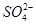 ��Cl����I����
��Cl����I����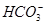 ��ȡ����Һ��������ʵ�飺
��ȡ����Һ��������ʵ�飺| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |
(1)��Һ��һ�����ڵ������� ����Һ�п϶������ڵ������� ��
(2)Ϊ��һ��ȷ���������ӣ�Ӧ�ò����ʵ�鼰��Ӧ���������ӵ�����(��Ϊ��Һ��Ӧ��˵��ʹ���Լ������ƣ�����д��ϸ����) ��
(3)д��ʵ��������з�Ӧ�����ӷ���ʽ�� ��
(1) ��
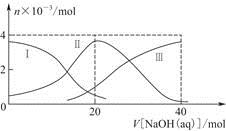
��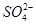 ��Mg2����Al3��Fe2����
��Mg2����Al3��Fe2����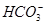 ��I����Ba2��
��I����Ba2��
(2)K���ļ����������ɫ��Ӧ��Cl���ļ��飺����Һ�м����������ᱵ��Һ�����˺�����Һ�м���������Һ���ټ�ϡ���ᣬ�����ɰ�ɫ�������ܽ⣬��֤����Cl��
(3)H����OH��=H2O Mg2����2OH��=Mg(OH)2�� Al3����3OH��=Al(OH)3�� Al(OH)3��OH��=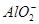 ��2H2O
��2H2O
 ��
��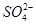 ��Mg2����Al3��Fe2����
��Mg2����Al3��Fe2����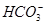 ��I����Ba2��
��I����Ba2��(2)K���ļ����������ɫ��Ӧ��Cl���ļ��飺����Һ�м����������ᱵ��Һ�����˺�����Һ�м���������Һ���ټ�ϡ���ᣬ�����ɰ�ɫ�������ܽ⣬��֤����Cl��
(3)H����OH��=H2O Mg2����2OH��=Mg(OH)2�� Al3����3OH��=Al(OH)3�� Al(OH)3��OH��=
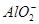 ��2H2O
��2H2Oԭ��Һ��ɫ�������в�����Fe2����ʵ���˵����Һ�����ԣ�����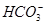 ���ܴ������ڣ�ʵ���˵����Һ�к���
���ܴ������ڣ�ʵ���˵����Һ�к��� ����ΪI���ܱ�HNO3����������I�����ܴ������ڣ�ʵ���˵��ԭ��Һ�к���
����ΪI���ܱ�HNO3����������I�����ܴ������ڣ�ʵ���˵��ԭ��Һ�к���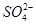 ����϶�����Ba2����ʵ�����˵��ԭ��Һ���Ƿ���Cl������Ϊʵ���������Cl����ʵ���˵����Һ�к���Mg2����Al3����
����϶�����Ba2����ʵ�����˵��ԭ��Һ���Ƿ���Cl������Ϊʵ���������Cl����ʵ���˵����Һ�к���Mg2����Al3����
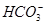 ���ܴ������ڣ�ʵ���˵����Һ�к���
���ܴ������ڣ�ʵ���˵����Һ�к��� ����ΪI���ܱ�HNO3����������I�����ܴ������ڣ�ʵ���˵��ԭ��Һ�к���
����ΪI���ܱ�HNO3����������I�����ܴ������ڣ�ʵ���˵��ԭ��Һ�к���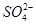 ����϶�����Ba2����ʵ�����˵��ԭ��Һ���Ƿ���Cl������Ϊʵ���������Cl����ʵ���˵����Һ�к���Mg2����Al3����
����϶�����Ba2����ʵ�����˵��ԭ��Һ���Ƿ���Cl������Ϊʵ���������Cl����ʵ���˵����Һ�к���Mg2����Al3����
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
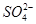
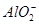 ��
��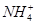 ��Na����
��Na����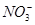
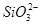
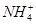 ��Fe2����Fe3����Ba2����
��Fe2����Fe3����Ba2����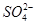 ��
��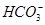 ��Cl���������������ʵ�飺���ò�����պȡ����Һ����pH��ֽ�ϣ���ֽ�Ժ�ɫ������ȡ������Һ����BaCl2��Һ�����ɲ�����ϡ����İ�ɫ��������ȡ�����ϲ���Һ�����ữ����������Һ��Ҳ���ɰ�ɫ���������й��ڸ���Һ��˵������ȷ����
��Cl���������������ʵ�飺���ò�����պȡ����Һ����pH��ֽ�ϣ���ֽ�Ժ�ɫ������ȡ������Һ����BaCl2��Һ�����ɲ�����ϡ����İ�ɫ��������ȡ�����ϲ���Һ�����ữ����������Һ��Ҳ���ɰ�ɫ���������й��ڸ���Һ��˵������ȷ����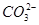 )��c(
)��c(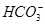 )
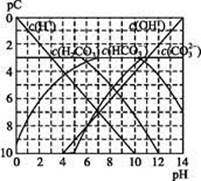
)
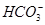 ����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)��
����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)�� 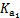 ������������
������������ 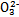 ��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ��
��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ�� 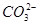 )="0.075" mol��L-1����t1ʱ����������ϵ�м���100 mL 0.125 mol��L-1 Na2CO3��Һ,��ʽ����˵���Ƿ��г���������
)="0.075" mol��L-1����t1ʱ����������ϵ�м���100 mL 0.125 mol��L-1 Na2CO3��Һ,��ʽ����˵���Ƿ��г���������