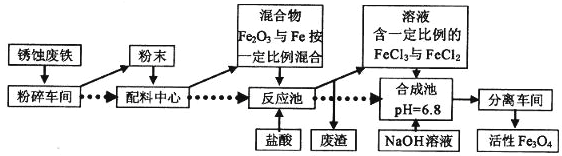
��Ŀ����
����Ŀ��25��ʱ���й����ʵĵ���ƽ�ⳣ�����£�
��ѧʽ | CH3COOH | H2CO3 | H2SO3 |
����ƽ�ⳣ�� | K=1.8��10��5 | K1=4.3��10��7 K2=5.6��10��11 | K1=1.5��10��2 K2=1.02��10��7 |
(1) CH3COOH��Һ�У������ƽ�ⳣ������ʽΪK =_____�������²��NaHSO3��Һ��pH��7����ԭ����_____��
(2) �����£������Ϊ10mLpH=2�Ĵ�����Һ����������Һ�ֱ������ˮϡ����1000mL��ϡ�ͺ���Һ��pH��ǰ��_____���ߣ����������������=������
(3) ��������CH3COO����CO32����HSO3����SO32������Һ�н��H���������ɴ�С�Ĺ�ϵΪ_____��
(4) �����ͬ��c(H��)��ͬ�Ģ�CH3COOH����HCl����H2SO4 ��������Һ�ֱ���ͬŨ�ȵ�NaOH��Һ��ȫ�к�ʱ������NaOH��Һ������ɴ�С������˳����_____(�����)��
(5) ��֪��H��(aq) + OH��(aq) == H2O(l) ��H =��57.3 kJ/mol��ʵ����ϡ������ϡNaOH��Һ��Ӧ����1 mol H2Oʱ�ų�57 kJ���ȣ��������Һ�У����������Ȼ�ѧ����ʽΪ_____��
���𰸡� ![]() HSO3���ĵ���̶ȴ�����ˮ��̶� �� CO32����SO32�� ��CH3COO����HSO3�� ��>��=�� CH3COOH(aq)
HSO3���ĵ���̶ȴ�����ˮ��̶� �� CO32����SO32�� ��CH3COO����HSO3�� ��>��=�� CH3COOH(aq) ![]() CH3COO��(aq) + H��(aq) ��H��+0.3 kJ/mol
CH3COO��(aq) + H��(aq) ��H��+0.3 kJ/mol
����������1��������뷽��ʽΪCH3COOH ![]() CH3COO�� + H��������ƽ�ⳣ������ʽΪK =
CH3COO�� + H��������ƽ�ⳣ������ʽΪK =![]() ����H2SO3�ĵ���ƽ�ⳣ����ˮ�ⳣ��=Kw/Ka�ɵã�NaHSO3��ҺHSO3-�ĵ������ˮ�⣬�ʳ�����NaHSO3��Һ��pH��7����2�����ڴ����������С�������ᣬpH��ͬ�Ĵ����������ϡ����ͬ�������������pH�仯�ʴ���pHС��������pH����3��KaԽС����Ӧ�������ӽ��H��������Խ�ʴ�Ϊ��CO32����SO32�� ��CH3COO����HSO3������4�����������pH��H2SO4��HCl�У�c��H+����ͬ���μӵ�Ũ�ȵ��������ƽ�����ǡ���кͣ���ȥ��������ȣ���CH3COOHΪ���ᣬ��pHʱ����Ũ�ȴ���HCl���μӵ�Ũ�ȵ��������ƽ�����ǡ���кͣ��������������������ӣ������ļ�࣬�ʢ�>��=�ۣ���5����ΪH��(aq) + OH��(aq) = H2O(l) ��H =-57.3 kJ/mol��CH3COOH(aq)+ OH��(aq) = H2O(l)+ CH3COO��(aq) ��H =-57. kJ/mol������CH3COOH(aq)
����H2SO3�ĵ���ƽ�ⳣ����ˮ�ⳣ��=Kw/Ka�ɵã�NaHSO3��ҺHSO3-�ĵ������ˮ�⣬�ʳ�����NaHSO3��Һ��pH��7����2�����ڴ����������С�������ᣬpH��ͬ�Ĵ����������ϡ����ͬ�������������pH�仯�ʴ���pHС��������pH����3��KaԽС����Ӧ�������ӽ��H��������Խ�ʴ�Ϊ��CO32����SO32�� ��CH3COO����HSO3������4�����������pH��H2SO4��HCl�У�c��H+����ͬ���μӵ�Ũ�ȵ��������ƽ�����ǡ���кͣ���ȥ��������ȣ���CH3COOHΪ���ᣬ��pHʱ����Ũ�ȴ���HCl���μӵ�Ũ�ȵ��������ƽ�����ǡ���кͣ��������������������ӣ������ļ�࣬�ʢ�>��=�ۣ���5����ΪH��(aq) + OH��(aq) = H2O(l) ��H =-57.3 kJ/mol��CH3COOH(aq)+ OH��(aq) = H2O(l)+ CH3COO��(aq) ��H =-57. kJ/mol������CH3COOH(aq) ![]() CH3COO��(aq) + H��(aq) ��H��+0.3 kJ/mol��
CH3COO��(aq) + H��(aq) ��H��+0.3 kJ/mol��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д�