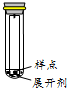
��Ŀ����
10������ݵ������Һ�����֪ʶ���ش��������⣺���������Ƶĵ��뷽��ʽNaOH=Na++OH-��0.1mol/L����������Һ��pH=13��
�ڴ��ᣨCH3COOH���ĵ��뷽��ʽCH3COOH?CH3COO-+H+��0.1mol/L������Һc��H+����0.1mol/L�������=����
����0.1mol/L������Һ�м��������ᣬ����ƽ�����棨��������桱����Ӧ�����ƶ���
����0.1mol/L������Һ�мӵ������Ũ�ȵ�����������Һ����Ӧ����Һ�Լ��ԣ�
���� ������������ǿ����ʣ���ȫ���룬����ˮ�����ӻ������������ӵ�Ũ�ȣ�
�ڴ���������ⲿ�ֵ��룬���뷽��ʽΪCH3COOH?CH3COO-+H+������0.1mol/L������Һc��H+��С��0.1mol/L��
����0.1mol/L������Һ�м��������ᣬ������Ũ�ȱ��ƽ�������ƶ���
����0.1mol/L������Һ�мӵ������Ũ�ȵ�����������Һ������ǡ����ȫ��Ӧ���ɴ�����ǿ�������Σ�ˮ����Һ�ʼ��ԣ�
��� �⣺������������ǿ����ʣ���ȫ���룬���뷽��ʽΪ��NaOH=Na++OH-������0.1mol/L����������Һ����������������ӵ�Ũ��Ϊ��0.1mol/L����C��H+��=$\frac{1��1{0}^{-14}}{0.1}$=10-13mol/L������PH=13���ʴ�Ϊ��NaOH=Na++OH-��13��
�ڴ���������ⲿ�ֵ��룬���뷽��ʽΪCH3COOH?CH3COO-+H+�����Դ�����������ʣ�0.1mol/L������Һc��H+��С��0.1mol/L���ʴ�Ϊ��CH3COOH?CH3COO-+H+������
����0.1mol/L������Һ�д���CH3COOH?CH3COO-+H+�����Լ��������ᣬ������Ũ�ȱ��ƽ�������ƶ����ʴ�Ϊ���棻
����0.1mol/L������Һ�мӵ������Ũ�ȵ�����������Һ������ǡ����ȫ��Ӧ���ɴ�����ǿ�������Σ�ˮ����Һ�ʼ��ԣ��ʴ�Ϊ���
���� ���⿼����ͼ�ĵ��������ϣ���ȷ��Ϻ�����Ũ�ȵĹ�ϵȷ����Һ���ʱ�������Ϊ���Ĺؼ���ע�����Ϊ���ᣬ��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ԭ�Ӱ뾶��С˳��r��W����r��Y����r��X�� | |
| B�� | �ǽ�����ǿ��˳��X��W��Z | |
| C�� | ����������Ӧˮ��������ԣ�Z��W | |
| D�� | ������Y2X��ZX2��WX3�л�ѧ��������ͬ |
| A�� | CuS | B�� | SO2 | C�� | FeCl3 | D�� | FeS |
| A�� | ʯ�͡���Ȼ�� | B�� | �˺���ˮ | C�� | ��ˮ | D�� | �ơ��ء�þ���� |
| A�� | �����ȴʡ�PM2.5����ָ������ֱ��С�ڻ����2.5 �ף�1��=1��10-6�ף��Ŀ�������PM2.5��������γɵķ�ɢϵ���ڽ��� | |
| B�� | ʳ���м��������⻯�أ�ʳ�ú�ɲ��������ڵ�Ԫ�صIJ��� | |
| C�� | ʯ�ʹ��ѻ�����ҪĿ����������͵������͵IJ�����������ʯ���ѽ����ҪĿ���ǵõ��������ϩ����ϩ����̬������ | |
| D�� | �������ͨ�Ź������������ά����Ҫԭ�� |