��Ŀ����
����Ŀ����֪��
��CO(g)��1/2O2(g)===CO2(g)����H����283.0 kJ��mol��1
��H2(g)��1/2O2(g)===H2O(g)����H����241.8 kJ��mol��1
����˵����ȷ����(����)
A. ͨ��״���£�������ȼ����Ϊ241.8 kJ��mol��1
B. �ɢٿ�֪��1 mol CO(g)��1/2 mol O2(g)��Ӧ���� 1 mol CO2(g)���ų�283.0 kJ������
C. ������ͼ��ʾ2CO2(g)===2CO(g)��O2(g)��Ӧ�����е������仯��ϵ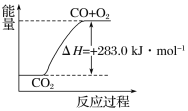
D. �ֽ�1 mol H2O(g)���䷴Ӧ��Ϊ��241.8 kJ
���𰸡�B
��������
A��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ���������ʱ�ų���������ˮ���ȶ�״̬ΪҺ̬����ȼ���Ȳ���241.8 kJ��mol��1��ѡ��A����B���Ȼ�ѧ����ʽ�е�ϵ����ʾ���ʵ���������CO(g)��1/2O2(g)===CO2(g)����H����283.0 kJ��mol��1�ĺ���Ϊ1 mol CO(g)��1/2 mol O2(g)��Ӧ���� 1 mol CO2(g)���ų�283.0 kJ��������ѡ��B��ȷ��C����ӦΪ���ȷ�Ӧ����ͼ���ʾ��Ϊ���ȷ�Ӧ��ѡ��C����D����Ӧ����������෴����Ӧ�ȵķ��Ÿı䣬���Էֽ�1 mol H2O(g)���䷴Ӧ��Ϊ+241.8 kJ��ѡ��D����ѡB��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�