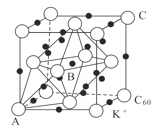
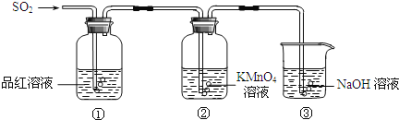
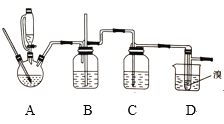
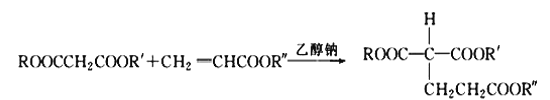��Ŀ����
����Ŀ�������ת������Դ���úͻ�����������Ҫ�о����⡣��H2S�Ϳ����Ļ������ͨ��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת����ͼ��ʾ������˵��������ǣ� ��

A.��ͼʾ��ת���У�Fe3+��CuS���м����
B.��ͼʾ��ת���У����ϼ۲����Ԫ��ֻ��ͭ
C.ͼʾת�����ܷ�Ӧ��2H2S+O2![]() 2S+2H2O
2S+2H2O
D.����1molH2Sת��Ϊ����ʱ����Ҫ����O2�����ʵ���Ϊ0.5mol
���𰸡�B
��������
A���ù����з�����Ӧ��Cu2++H2S��CuS+2H+��CuS+Fe3+��S+Fe2++Cu2+(δ��ƽ)��Fe2++O2��Fe3+(δ��ƽ)���ɴ˿�֪��Fe3+��CuS���м�����A���������⣻
B����ͼ֪�����ϼ۱仯��Ԫ���У�S��Fe��O��Cu��H��Cl�Ļ��ϼ�û�����仯����B�������⣻
C����Aѡ����������������ԭ��Ӧת�Ƶ����غ㡢ԭ���غ��֪���䷴Ӧ���ܷ�ӦΪ��2H2S+O2![]() 2S+2H2O����C���������⣻
2S+2H2O����C���������⣻
D��H2S��Ӧ����S����Ԫ�ػ��ϼ�����2�ۣ�O2��Ӧʱ��Ԫ�ػ��ϼ۽���2������������ԭת�Ƶ����غ��֪������1molH2Sת��Ϊ����ʱ����Ҫ����O2�����ʵ���Ϊ0.5mol����D���������⣻
�ʴ�Ϊ��B��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����(1)��״���£�1.92 gij��������Ϊ672 mL������������Է�������Ϊ________��
(2)��25 �桢101 kPa�������£�ͬ������CH4��A��������֮����15��8����A��Ħ������Ϊ______________��
(3)������ͬ�ݻ����ܱ�����X��Y����25 ���£�X�г���a g A���壬Y�г���a g CH4���壬X��Y�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ________��
���ڱ�״���£���aLNH3��ȫ����ˮ�õ�VmL��ˮ����Һ���ܶ�Ϊ�� g��cm��3���������Һ��Ũ��(��������ĸ��ʾ)��
(1)����Һ�����ʵ���Ũ��________________
(2)����Һ����������_________________________
����������Һ����������������ͬ������Ħ������ΪM g��mol��1�������±���Ϣ���ش��й����⣺
���ʵ��������� | ��Һ���ܶ�(g��cm��3) | |
��һ����Һ | w1 | ��1 |
�ڶ�����Һ | w2 | ��2 |
(1)ȡ������������Һ��ϣ������û��Һ�����ʵ���������w3��________��(��������ĸ��ʾ)
(2)����w1>w2��ȡ�������������Һ��ϣ������û��Һ�����ʵ���������Ϊw4������1>��2����w3___w4������1<��2��w3______w4(����>����<������=��)��