��Ŀ����
12������������Ԫ��X��Y��Z��W��M��Nԭ��������������X��M��W��N�ֱ�ͬ���壬X������Ԫ�ؾ���ͬ���ڣ�Y��Z��Wͬ�����������ڱ���λ�����ڣ���֪N�����������������������2������ش��������⣺��I��YԪ�������ڱ��е�λ���ǵڶ�����IVA�壬X��Y��Z��W��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��C��N��O��H����Ԫ�ط��ű�ʾ����
��2��д��X2W2����ʽ
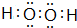 ��YW2������״Ϊֱ���Σ�
��YW2������״Ϊֱ���Σ���3��X��Z��W�γɵĻ������У�ˮ��Һһ�������Ե�����NH4NO3 ��д��ѧʽ������ԭ����NH4++H2O?NH3��H2O+H+ �������ӷ���ʽ��ʾ����������������ѧ�������������Ӽ������ۼ���
��4��Һ̬Z2X4��Һ̬Z2W4��Ӧ�����ﻷ������Ⱦ����֪�ڴ˷�Ӧ��16g Z2X4��ȫ��Ӧ������̬ˮʱ�ͷų�����260kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ2N2H4��l��+N2O4��g��=3N2��g��+4H2O��g����H=-1040kJ•mol-1��
��5����һ�����ӷ���ʽ��ʾNW2ͨ��Ba��NO3��2��Һ�еķ�Ӧ3Ba2++3SO2+2NO3-+2H2O=3BaSO4��+2NO��+4H+��
���� ����������Ԫ��X��Y��Z��W��M��Nԭ��������������W��Nͬ���壬���ԭ��������֪W���ڵڶ����ڡ�N���ڵ������ڣ���֪N�����������������������2������������Ϊ16����NΪSԪ�ء�WΪOԪ�أ���X��Mͬ���壬��X������Ԫ�ؾ���ͬ���ڣ���Xֻ�ܴ��ڵ�һ���ڣ���XΪHԪ�أ�M��ԭ����������������MΪNa��Y��Z��Wͬ�����������ڱ���λ�����ڣ���YΪCԪ�ء�ZΪNԪ�أ��ݴ˽��
��� �⣺����������Ԫ��X��Y��Z��W��M��Nԭ��������������W��Nͬ���壬���ԭ��������֪W���ڵڶ����ڡ�N���ڵ������ڣ���֪N�����������������������2������������Ϊ16����NΪSԪ�ء�WΪOԪ�أ���X��Mͬ���壬��X������Ԫ�ؾ���ͬ���ڣ���Xֻ�ܴ��ڵ�һ���ڣ���XΪHԪ�أ�M��ԭ����������������MΪNa��Y��Z��Wͬ�����������ڱ���λ�����ڣ���YΪCԪ�ء�ZΪNԪ�أ�
��1��YΪCԪ�أ������ڱ��е�λ���ǣ��ڶ�����IVA�壬����Ԫ����Hԭ�Ӱ뾶��С��ͬ�����������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��C��N��O��H��
�ʴ�Ϊ���ڶ�����IVA�壻C��N��O��H��
��2��H2O2����ʽΪ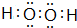 ��CO2������״Ϊֱ���Σ�
��CO2������״Ϊֱ���Σ�
�ʴ�Ϊ��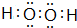 ��ֱ���Σ�
��ֱ���Σ�
��3��H��N��O�γɵĻ������У�ˮ��Һһ�������Ե����� NH4NO3����ԭ���ǣ�NH4++H2O?NH3��H2O+H+��������������ѧ���������У����Ӽ������ۼ���
�ʴ�Ϊ��NH4NO3��NH4++H2O?NH3��H2O+H+�����Ӽ������ۼ���
��4��Һ̬N2H4��Һ̬N2O4��Ӧ�����ﻷ������Ⱦ����Ӧ����N2��H2O��������Ӧ��2N2H4+N2O4=3N2+4H2O
����֪�ڴ˷�Ӧ��16g N2H4��ȫ��Ӧ������̬ˮʱ�ͷų�����260kJ����2molN2H4��Ӧ�ų�������Ϊ260kJ��$\frac{2mol��32g/mol}{16g}$=1040kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ��2N2H4��l��+N2O4��g��=3N2��g��+4H2O��g����H=-1040kJ•mol-1 ��
�ʴ�Ϊ��2N2H4��l��+N2O4��g��=3N2��g��+4H2O��g����H=-1040kJ•mol-1��
��5��SO2ͨ��Ba��NO3��2��Һ�У����ɵ������ᣬ��Һ�����ԣ����������������������ǿ�����ԣ�������������Ϊ���ᣬ������뱵���ӷ�Ӧ�������ᱵ��������Ӧ���ӷ���ʽΪ��3Ba2++3SO2+2NO3-+2H2O=3BaSO4��+2NO��+4H+��
�ʴ�Ϊ��3Ba2++3SO2+2NO3-+2H2O=3BaSO4��+2NO��+4H+��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ������ضԻữѧ����Ŀ��飬ע��Ի���֪ʶ���������գ�

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�| A�� | 18Oԭ������������Ϊ8 | |
| B�� | 1mol��1H218O��������������Ϊ12�� | |
| C�� | 18O2�����Ħ�����Ϊ22.4L/mol | |
| D�� | 18O2�����Ħ������Ϊ36g/mol |
| A�� | K+��H+��Cl-��OH- | B�� | K+��H+��HCO3-��Cl- | ||
| C�� | Fe2+��H+��Br-��OH- | D�� | K+��NH4+��SO42-��Cl- |
| A�� | NH3 | B�� | SO3 | C�� | ���� | D�� | H2SO4 |
���ܱ������н�����CuBr2��487K�¼��ȷֽ⣬ƽ��ʱp��Br2��Ϊ4.66��103Pa��
�練Ӧ�¶Ȳ��䣬����Ӧ��ϵ���������һ������p��Br2���ı仯��ΧΪ��������
| A�� | p��Br2����4.66��103Pa | B�� | 2.33��103Pa��p��Br2����4.66��103Pa | ||
| C�� | ��p��Br2����2.33��103Pa | D�� | 2.33��103Pa��p��Br2����4.66��103Pa |
| A�� | ����������������ɢϵ�ı��������Ƿ�ɢ�ʵ���ֱ����10-9��10-7m֮�� | |
| B�� | һ������������ʱ�������пɷ��������ЧӦ | |
| C�� | ����ķ�ɢ������ֱ������Һ�Ĵʽ�����������ֽ | |
| D�� | Fe��OH��3�����ܹ�ʹˮ�������Ĺ�������������ﵽ��ˮĿ�� |
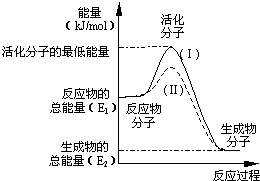 �ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӳ�Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ/mol��ʾ��������۲���ͼ��Ȼ��ش����⣺
�ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӳ�Ϊ����ӣ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ/mol��ʾ��������۲���ͼ��Ȼ��ش����⣺