��Ŀ����
16�������йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | ���ʵ���Ũ����ȵĢ٣�NH4��2CO3�ڣ�NH4��2SO4�ۣ�NH4��2Fe��SO4��2������Һ��c��NH4+���Ĵ�С˳��Ϊ���٣��ڣ��� | |
| B�� | ��0.1mol•L-1Na2CO3��Һ�У�c��OH-��-c��H+��=c��HCO3-��+2c��H2CO3�� | |
| C�� | ��0.2mol•L-1NaHCO3��Һ�м�������0.1mol•L-1NaOH��Һ��c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| D�� | 0.2mol•L-1HCl��0.1mol•L-1NaAlO2��Һ�������ϣ�c��Cl-����c��Na+����c��Al3+����c��H+����c��OH-�� |
���� A��̼������Ӵٽ�笠�����ˮ�⡢������������笠�����ˮ�⣬笠�����ˮ��̶�Խ����Һ��c��NH4+��ԽС��
B������̼������Һ�е������غ��жϣ�
C����Ӧ������Ϊ��Ũ�ȵ�̼�����ƺ�̼���ƣ�̼������ӵ�ˮ��̶ȴ���̼��������ӣ���c��HCO3-����c��CO32-����
D.0.2mol/LHCl��0.1mol/L NaAlO2��Һ�������ϣ��������Ϊ1L����Ӧ������Al��OH��3��Al3+�����жϸ�����Ũ�ȴ�С��
��� �⣺A���٣�NH4��2CO3̼������Ӵٽ�笠�����ˮ��ۣ�NH4��2Fe��SO4��2������������笠�����ˮ�⣬����笠�����ˮ��̶�Խ����Һ��c��NH4+��ԽС������c��NH4+���ɴ�С��˳���Ǣۣ��ڣ��٣���A����
B����0.1mol•L-1Na2CO3��Һ�У����������غ�ɵã�c��OH-��=c��H+��+c��HCO3-��+2c��H2CO3������c��OH-��-c��H+��=c��HCO3-��+2c��H2CO3������B��ȷ��
C����0.2mol•L-1NaHCO3��Һ�м�������0.1mol•L-1NaOH��Һ����Ӧ������Ϊ��Ũ�ȵ�̼�����ƺ�̼���ƣ�̼������ӵ�ˮ��̶ȴ���̼��������ӣ���c��HCO3-����c��CO32-��������ȷ������Ũ�ȴ�СΪ��c��HCO3-����c��CO32-����c��OH-����c��H+������C����
D.0.2mol/LHCl��0.1mol/L NaAlO2��Һ�������ϣ��������Ϊ1L����Ӧ������Al��OH��3��Al3+������Al3+Ϊ$\frac{1}{3}$mol��Al3+ˮ������ԣ�����Һ������Ũ�ȴ�С˳��Ϊ��c��Cl-����c��Na+����c��Al3+����c��H+����c��OH-������D��ȷ��
��ѡBD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�ע�������ε�ˮ��ԭ������Ӧ�÷�������ȷ����غ㡢�����غ㼰�ε�ˮ�����ж�����Ũ�ȴ�С�е�Ӧ�ã�����������ѧ���ķ������������Ӧ��������

| A�� | c=$\frac{3b}{0.0224V}$ | B�� | e=a+$\frac{8cV}{1000}$ | C�� | d=a+$\frac{17cV}{1000}$ | D�� | $\frac{82a}{65}$��e��$\frac{5a}{4}$ |
| A�� | ��ϩ����֬����Ȼ�͵����ʷ����о�����˫�� | |
| B�� | ��ͬ���ʵ����ļ����ǻ��������ĵ�����Ŀ��ͬ | |
| C�� | ���³�ѹ�£�3.0g�����Ǻͱ�����Ļ�����к��е�ԭ��������Ϊ��ֵ | |
| D�� | ���ú�������Ǽ�¼�ĺ������ͼ���Գ����Ʋ��л��������й����ŵ���� |
| A�� | ��AB2�͵Ĺ��ۻ����������ԭ��A������sp�ӻ�����ɼ� | |
| B�� | 25��ʱ����Mg��OH��2������Һ�м���������NH4Cl���壬c��Mg2+������ | |
| C�� | ��Ӧ2A��g��+B��g���T3C��s��+D��g����һ�����������Է����У�˵���÷�Ӧ�ġ�H��0 | |
| D�� | SO2��SiO2�ľ����У���ѧ������;������;���ͬ |
| A�� | Ca��HCO3��2 | B�� | Na2SiO3 | C�� | FeCl2 | D�� | AlCl3 |
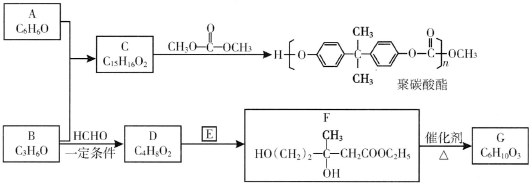
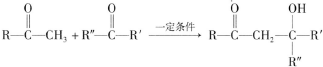 ��R��R�䡢R��Ϊ��ԭ�ӻ�������
��R��R�䡢R��Ϊ��ԭ�ӻ�������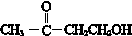 ��E�ķ���ʽ��C4H8O2��F����G�ķ�Ӧ������ȡ����Ӧ��
��E�ķ���ʽ��C4H8O2��F����G�ķ�Ӧ������ȡ����Ӧ��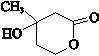 ��G��ͬ���칹���ж��֣����к�G������ȫ��ͬ��״�ṹ��ͬ���칹����23�֣�������G�������������칹����
��G��ͬ���칹���ж��֣����к�G������ȫ��ͬ��״�ṹ��ͬ���칹����23�֣�������G�������������칹����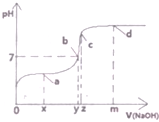 ��1��25��ʱ����20mL0.1mol•L�Ĵ�����Һ�в��ϵ���0.1mol•L-1��NaOH��Һ����Һ��pH�仯������ͼ��ʾ��
��1��25��ʱ����20mL0.1mol•L�Ĵ�����Һ�в��ϵ���0.1mol•L-1��NaOH��Һ����Һ��pH�仯������ͼ��ʾ��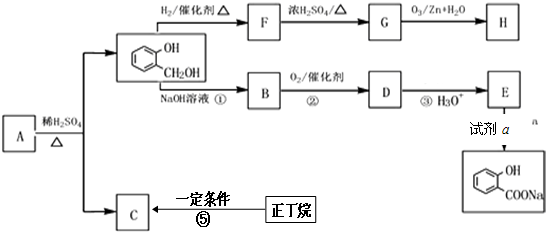
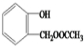 ����F��G�ķ�Ӧ����Ϊ��ȥ��Ӧ��
����F��G�ķ�Ӧ����Ϊ��ȥ��Ӧ�� ��
��
 �ķ�Ӧ�Ƿ������ȫ�����һ����ʵ�����֤����д��ʵ������������ۣ�ȡ������Ӧ�����Һ���Թ��У������Ȼ�����Һ������Һ����ɫ����Ӧ����ȫ����Ӧ����ʣ�࣬��֮����ȫ��Ӧ��
�ķ�Ӧ�Ƿ������ȫ�����һ����ʵ�����֤����д��ʵ������������ۣ�ȡ������Ӧ�����Һ���Թ��У������Ȼ�����Һ������Һ����ɫ����Ӧ����ȫ����Ӧ����ʣ�࣬��֮����ȫ��Ӧ�� C��${\;}_{6}^{12}$C��${\;}_{6}^{14}$C D������ͱ��飮
C��${\;}_{6}^{12}$C��${\;}_{6}^{14}$C D������ͱ��飮