��Ŀ����
����Ŀ����ʯ�ͺ͵��ۻ���ά��Ϊԭ�Ͼ����Ƶ�����������ת����ϵ����ͼ��
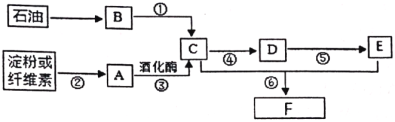
��������B�ڱ���µ��ܶ�Ϊ��1.25 g/L����ش�
��1��B�й����ŵ�������________��
��2��A�ķ���ʽ��________��
��3����Ӧ�ܵĻ�ѧ����ʽΪ________��
��4������˵����ȷ����________��
A����Ӧ�ڢݢ�����ȡ����Ӧ
B������F�Ľṹ��ʽΪCH3CH2OOCCH3
C��B��E����һ��������ֱ�Ӻϳ�F���÷���������ɫ��ѧ����
D����������������ͭ����C��D��E��������
���𰸡�̼̼˫�� C6H12O6 2CH3CH2OH + O2![]() 2CH3CHO +2H2O BCD
2CH3CHO +2H2O BCD
��������
����B�ڱ���µ��ܶ�Ϊ��1.25 g/L����Ħ������ = 1.25 g/L��22.4 L/mol = 28 g/mol����������B������ʯ���Ƶã�����Ƴ�����BΪ��ϩ�����ݷ�Ӧ�ڿ�֪����AΪ�����ǣ������ƻ�ø���û������Ҵ�����CΪ�Ҵ����Ҵ�������Ӧ�ܱ���������ȩ����DΪ��ȩ������������������Ӧ�ݣ��������ᣬ��EΪ���ᣬ��ȩ��������һ�������·���������Ӧ����������������FΪ�����������ݴ˷�������
����������������
��1��BΪ��ϩ��������ڹ���������Ϊ̼̼˫����
�ʴ�Ϊ̼̼˫����
��2��AΪ�����ǣ������ʽΪC6H12O6��
�ʴ�Ϊ��C6H12O6��
��3����Ӧ��Ϊ�Ҵ���ͭ�������ȵ������±�������ȩ�Ĺ��̣����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH + O2![]() 2CH3CHO +2H2O��
2CH3CHO +2H2O��
�ʴ�Ϊ��2CH3CH2OH + O2![]() 2CH3CHO +2H2O��
2CH3CHO +2H2O��
��4��A. ��Ӧ��Ϊ���۵�ˮ�ⷴӦ������ȡ����Ӧ����Ӧ��Ϊ��ȩ������Ϊ����Ĺ��̣�����������Ӧ����Ӧ��Ϊ����������������Ӧ������ȡ����Ӧ����A�����
B. ����F��Ϊ����������Ϊ�������Ҵ�ͨ�������ǻ�������õ�����Ҫ�������ӵĽṹ��ʽΪCH3CH2OOCCH3����B����ȷ��
C. BΪ��ϩ��EΪ���ᣬ����ֱ�ӻ��ϣ������ԭ��������Ϊ100%��������ɫ��ѧ�����C����ȷ��
D. ����������ͭ���Ҵ����ܣ�����ȩ�ᷢ����Ӧ����ש��ɫ�����������ᷢ������кͣ�����Һ����ɫ������Һ�����������������ͭ����C��D��E�������ʣ���D����ȷ��
��ѡB��C��D��

����Ŀ��A��B��C��D��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е����λ����ͼ��ʾ������B�ĵ����ڿ����к���Լռ80����
A | B | C | |||
D |
��1��д������Ԫ�ص����ƣ�C____��D___��
��2������B��ԭ�ӽṹʾ��ͼ____��C��Ԫ�����ڱ��е�λ����____��
��3��B��C����Ԫ������⻯����ȶ�����ǿ������˳����_____��д��A������⻯��ĵ���ʽ��______��






