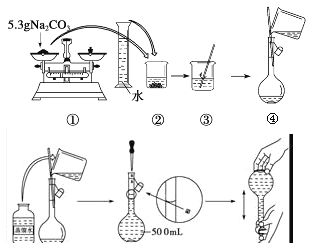
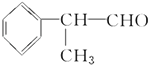
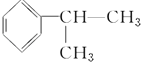
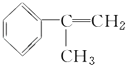
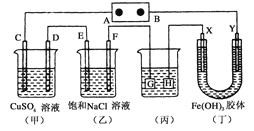
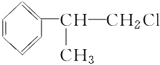��Ŀ����
����Ŀ��ij��ɫ��Һ�к���K+��Cl����OH����SO![]() ��SO
��SO![]() ��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
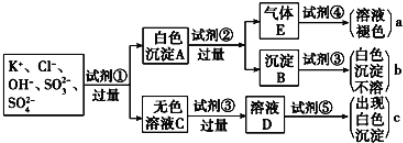
��1��ͼ���Լ����������ʵĻ�ѧʽ�ֱ���
��________����________����________����__________����__________��
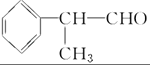
��2��ͼ������a��b��c��������������ӷֱ���a________��b________��c________��
��3����ɫ����A���Լ�����Ӧ�����ӷ���ʽ_________________��
��4����ɫ��ҺC���Լ�������ҪĿ����_____________________��
��5����ɫ����A�����Լ����������Լ�������ʵ���Ӱ����____________________��
��6������Eͨ���Լ���������Ӧ�����ӷ���ʽ��____________________��
���𰸡�
��1��Ba(NO3)2��HCl��HNO3��Br2��AgNO3��
��2��SO32-��SO42-��Cl-��
��3��BaSO3+2H+=Ba2++SO2��+H2O��
��4���к�OH-����ֹ��Cl-�ļ���������ţ�
��5����ʹSO32-��SO42-�ļ���������ţ�����ȷ��SO42-��SO32-�Ƿ���ڣ�
��6��Br2+SO2+2H2O=4H++SO42-+2Br-��
��������
�����������1��SO32-��SO42-��Ba(NO3)2��Һ��Ӧ�ֱ����������ᱵ�����ᱵ��ɫ�����������ᱵ�����ᷴӦ���ɶ����������壬������������E��ʹ��ˮ��ɫ�����ᱵ���ܽ��������У����Լ���ΪBa(NO3)2��Һ���Լ���Ϊ��������ᣬ�Լ���Ϊ��ˮ����ɫ��ҺC�ʼ��ԣ���������Լ������������Һ�����ԣ��ټ����Լ�����������Һ�������Ȼ�����ɫ�������ʴ�Ϊ��Ba(NO3)2��HCl��HNO3��Br2��AgNO3��
��2��SO32-��SO42-��Ba(NO3)2��Һ��Ӧ�ֱ����������ᱵ�����ᱵ��ɫ�����������ᱵ�����ᷴӦ���ɶ����������壬����������ʹ��ˮ��ɫ����Ӧ����ʽ��SO2+Br2+2H2O=H2SO4+2HBr�����ᱵ���ܽ⣬���Լ���ΪBa(NO3)2��Һ���ɴ˿��Ʋ��Լ���Ϊij�ᣬ�������ᱵ��Ӧ��SO2���壬���Ǻ�����Ҫ���SO42-�������������ὫSO32-������Ӱ���SO42-���жϣ���ӦΪ���ᣬ�Լ���Ϊ��ˮ����������a�������������ΪSO32-������b�������������ΪSO42-����ɫ��ҺC�ʼ��ԣ���������Լ������������Һ�����ԣ��ټ����Լ�����������Һ�������Ȼ�����ɫ������������c�������������ΪCl-���ʴ�Ϊ��SO32-��SO42-��Cl-��
��3�������ᱵ���Ժ�ǿ�ᷴӦ���ɿ����Եı��κ�ˮ�Լ���������BaSO3+2H+�TBa2++SO2��+H2O���ʴ�Ϊ��BaSO3+2H+=Ba2++SO2��+H2O��
��4����ɫ��ҺA�к���OH-��OH-����������Ӧ������������ɫ���������Ŷ�Cl-�ļ��飬���Լ������ϡ���ᣬ�к�OH-����ֹ��Cl-�ļ���������ţ��ʴ�Ϊ���к�OH-����ֹ��Cl-�ļ���������ţ�
��5����ɫ����A�����Լ���ϡ����������Լ�����ʹSO32-��SO42-�ļ���������ţ�����ȷ��SO42-�Ƿ���ڣ��ʴ�Ϊ����ʹSO32-��SO42-�ļ���������ţ�����ȷ��SO42-��SO32-�Ƿ���ڣ�
��6���嵥�����������Ӧ��������������ᣬ���ӷ���ʽ��Br2+SO2+2H2O=4H++SO42-+2Br-���ʴ�Ϊ��Br2+SO2+2H2O=4H++SO42-+2Br-��