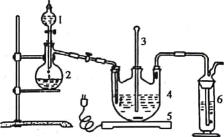
��Ŀ����
����Ŀ����һ���¶��£�10 mL 4.0 mol/L H2O2�����ֽ⣺2H2O2![]() H2O+ O2
H2O+ O2![]() ����ͬʱ�̲ⶨ����O2�������������Ϊ��״�������±���
����ͬʱ�̲ⶨ����O2�������������Ϊ��״�������±���
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
����������ȷ���ǣ���Һ����仯���Բ��ƣ�
A. 6 minʱ��Ӧ������(H2O2) = 3.33��10-2 mol/(L��mol)
B. ��Ӧ��6 minʱ��H2O2�ֽ���Ϊ50%
C. �����������䣬�����ø���Ч�Ĵ������ﵽƽ��ʱ����õ���������
D. ��ʱ�������õ����������࣬˵����Ӧ�����ӿ�
���𰸡�B
��������
A. ��Ӧ������ƽ����Ӧ���ʣ�
B. ����ת���� = ![]() ��
��
C. ���������ƽ�ⲻ�ƶ���
D. ����ʱ����У���Ӧ��Ũ�Ƚ��ͣ���Ӧ���ʱ�С��
A. ��ѧ��Ӧ����ָ����ƽ����Ӧ���ʣ�����˲ʱ���ʣ���������6 minʱ��Ӧ���ʣ���A�����
B. ��Ӧ��6 minʱ��V(O2) = 22.4 mL����������ʵ���n(O2) = ![]() = 10-3 mol�����ݹ�ϵʽ2H2O2
= 10-3 mol�����ݹ�ϵʽ2H2O2![]() O2��֪��ת����n(H2O2) = 2 n(O2) = 2��10-3 mol������ʼ�Ĺ�����������ʵ���Ϊ10��10-3 L ��4.0 mol/L = 4��10-3 mol���ʷֽ��� =
O2��֪��ת����n(H2O2) = 2 n(O2) = 2��10-3 mol������ʼ�Ĺ�����������ʵ���Ϊ10��10-3 L ��4.0 mol/L = 4��10-3 mol���ʷֽ��� = ![]() 100% = 50%����B����ȷ��
100% = 50%����B����ȷ��
C. �����������䣬�����ø���Ч�Ĵ�������ƽ�ⲻ�ƶ�����õ�������������C�����
D. ��ʱ�������õ����������࣬˵����Ӧһֱ���У�����Ӧ����������Ũ��Խ��Խ�ͣ�˵����Ӧ�������ͣ���D�����
��ѡB��