��Ŀ����
����Ŀ������![]() ��Һ��
��Һ��![]() ��Һ��Ӧ�����ӷ���ʽΪ��
��Һ��Ӧ�����ӷ���ʽΪ��![]() ��ijͬѧ������±���ʾ����̽���¶ȡ�Ũ�ȸı�Ը÷�Ӧ���ʵ�Ӱ�������
��ijͬѧ������±���ʾ����̽���¶ȡ�Ũ�ȸı�Ը÷�Ӧ���ʵ�Ӱ�������
ʵ����� | ʵ���¶� | �μӷ�Ӧ������ | ��Һ��ɫ������ɫ����ʱ�� | ||||
|
|
| |||||
|
|
|
|
| |||
A | 298 | 2 |
| 4 | a | 0 |
|
B |
| 2 |
| 3 | a |
| 10 |
C | 318 | 2 |
|
| a | 1 |
|
��1��ʵ����a����СֵΪ____________��̽���¶ȱ仯�Է�Ӧ����Ӱ�����������ʵ����________��
��2��![]() ____________��
____________��![]() ____________������ʵ��B�����ݼ��㣬��
____________������ʵ��B�����ݼ��㣬��![]() ��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ
��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ![]() ________��
________��
��3�����Ƿ��֣�A��ʵ�����ռ���![]() �����ͼa��ʾ����ʹA�鷴Ӧ�ھ��������н��У��÷�Ӧ�ķ�Ӧ��������ʱ��ı仯��ͼb��ʾ��
�����ͼa��ʾ����ʹA�鷴Ӧ�ھ��������н��У��÷�Ӧ�ķ�Ӧ��������ʱ��ı仯��ͼb��ʾ��
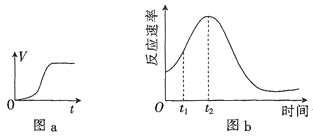
![]() ʱ�������ʱ�����Ҫԭ������ǣ�һ�Dz�����
ʱ�������ʱ�����Ҫԭ������ǣ�һ�Dz�����![]() �Ƿ�Ӧ�Ĵ���������______________________________��
�Ƿ�Ӧ�Ĵ���������______________________________��
![]() ��ͼb��Ϣ֪�������Ĵ�Ч����������________
��ͼb��Ϣ֪�������Ĵ�Ч����������________![]() �����й���������������ȷ����
�����й���������������ȷ����![]() ��
��
���𰸡�![]() B��C
B��C ![]() 298
298 ![]() �÷�Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ��Һ�¶����� �й�
�÷�Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ��Һ�¶����� �й�
��������
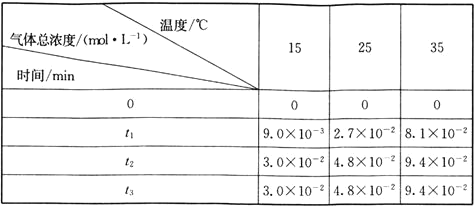
�о�ij�����ضԷ�Ӧ���ʵ�Ӱ��ʱ����Ҫ��֤����������ͬ����Ӧ���ÿ��Ƶ�һ����������̽�����������������ݿ�֪��A��B�в���������ͬ����A��B̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬��A��BӦ�����¶���ͬ��![]() ��Ϊʹ
��Ϊʹ![]() ��ҺŨ���ڼ���ʵ������ȣ���A��֪����ʵ���л����Һ���������Ϊ6mL����VB=1mL��VC=3mL��B��C�¶Ȳ�ͬ����̽���¶ȶԷ�Ӧ���ʵ�Ӱ�죬�ݴ˷������
��ҺŨ���ڼ���ʵ������ȣ���A��֪����ʵ���л����Һ���������Ϊ6mL����VB=1mL��VC=3mL��B��C�¶Ȳ�ͬ����̽���¶ȶԷ�Ӧ���ʵ�Ӱ�죬�ݴ˷������
![]() Ϊʹ
Ϊʹ![]() ��Һ��ɫ��ȫ��ʧ��������ҺӦ������������й�ϵʽ��
��Һ��ɫ��ȫ��ʧ��������ҺӦ������������й�ϵʽ��![]() ���ɵ�
���ɵ�![]() �����
�����![]() ��ʵ��B��CŨ����ͬ���¶Ȳ�ͬ����̽���¶ȱ仯�Է�Ӧ����Ӱ��������ʴ�Ϊ��
��ʵ��B��CŨ����ͬ���¶Ȳ�ͬ����̽���¶ȱ仯�Է�Ӧ����Ӱ��������ʴ�Ϊ��![]() ��B��C��
��B��C��
![]() ��
��![]() ������ʵ��B�����ݼ��㣬
������ʵ��B�����ݼ��㣬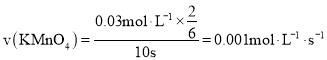 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��298��
��298��![]() ��
��
![]() ��Ӧ��ʼ��Ӧ��Ũ�ȼ�С���ʵ�����Һ�з�Ӧ���ʼӿ��ԭ��һ�Dz�����
��Ӧ��ʼ��Ӧ��Ũ�ȼ�С���ʵ�����Һ�з�Ӧ���ʼӿ��ԭ��һ�Dz�����![]() �Ƿ�Ӧ�Ĵ��������Ǹ÷�Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ��Һ�¶����ߣ��ʴ�Ϊ���÷�Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ��Һ�¶����ߣ�
�Ƿ�Ӧ�Ĵ��������Ǹ÷�Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ��Һ�¶����ߣ��ʴ�Ϊ���÷�Ӧ�Ƿ��ȷ�Ӧ���ų�������ʹ��Һ�¶����ߣ�
![]() ��Ӧ��ʼ�������ͻ���
��Ӧ��ʼ�������ͻ���![]() �γɣ��������Ӧ�������Ӳ����ԣ�˵����Ч������������йأ��ʴ�Ϊ���йء�
�γɣ��������Ӧ�������Ӳ����ԣ�˵����Ч������������йأ��ʴ�Ϊ���йء�

 ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д�����Ŀ��Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���֪��ˮ��ȡþ����Ҫ������ͼ��
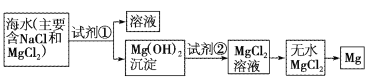
��1�����ڼ����Լ������������������¼��ֲ�ͬ������������������⡣
���� | �Ƿ���ȷ | �������� |
����1��ֱ������ˮ�м�������� | ����ȷ | ��ˮ��þ����Ũ��С�������������������� |
����2�����¼���������ˮ���ټ�������� | ����ȷ | ��һ�� |
����Ϊ����������������ǣ������� | ||
��һ��___��
������___��
��2����ͼ�м�����Լ���Ӧ����___���ѧʽ����������Լ�����___���ѧʽ������ҵ������ˮMgCl2��ȡþ�Ļ�ѧ����ʽΪ___��
��3�������Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����___��
����Ŀ����ʽ������(NiOOH)�����������ص��������ϣ����÷�����������Ҫ��Ni��Al������Cr��FeS ��)���Ʊ����乤���������£�

�ش��������⣺
(1)�����ݳ�����ʱ��������Ӧ�����ӷ�Ӧ����ʽΪ_________________________;
(2)���ܽ⡱ʱ�ų�������Ϊ_______________ (�ѧʽ);
(3)��֪�������½������ӿ�ʼ��������ȫ������pH���±���
��ʼ������pH | ��ȫ������pH | |
Ni2+ | 6.2 | 8.6 |
Fe2+ | 7.6 | 9.1 |
Fe3+ | 2.3 | 3.3 |
Cr3+ | 4.5 | 5.6 |
����pH 1��ʱ����ҺpH��ΧΪ______________________��
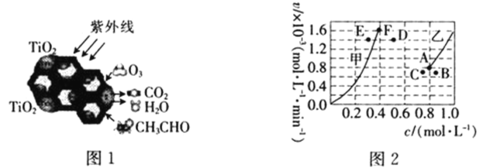
(4)�ڿ����м���Ni(OH)2�ɵ�NiOOH,��д���˷�Ӧ�Ļ�ѧ����ʽ_____________;

(5)����������Һ���ж��ִ�����ʽ, CrO42����Cr2O72������Һ�п��ת���������£���ʼŨ��Ϊ1.0mol/L��Na2CrO4��Һ��c(Cr2O72��)��c(H+)�ı仯��ͼ��ʾ�������ӷ���ʽ��ʾNa2CrO4��Һ�е�ת����Ӧ________________������A�����ݼ������ת����Ӧ��ƽ�ⳣ��Ϊ______________,�¶����ߣ���Һ��CrO42����ƽ��ת���ʼ�С����÷�Ӧ�ġ�H____0���>������<����=������