جâؤ؟ؤعبف
¹¤زµةد½«ضئب،ضط¸ُثل¼ط(K2Cr2O7)؛ح¸ُثلôû(CrO3)µؤت£سà·دشü³ئخھ¸ُشü£¬ئن³ة·ضخھSiO2،¢Al2O3،¢MgO،¢Fe2O3،¢CrO3،¢K2Cr2O7µب،£
زرضھ£؛
¢ظضط¸ُثل¼ط؛ح¸ُثلôû¶¼ز×بـسعث®£¬صâتاشى³ة¸ُخغب¾µؤض÷زھشزٍ£¬ثüأا¶¼تاا؟رُ»¯¼ء£¬¸ُثلôûبـسعث®³تثلذش£»
¢ع£«6¼غ¸ُز×±»بثجهخüتص£¬؟ةضآ°©£»£«3¼غ¸ُ²»ز×±»بثجهخüتص£¬¶¾ذشذ،،£
¢غدآ±يخھز»ذ©½ًتôاâرُ»¯خï³ءµيµؤpH²خصصت¾ف،£
| خïضت | ؟ھت¼³ءµي | حêب«³ءµي |
| Fe(OH)3 | 2.7 | 3.7 |
| Al(OH)3 | 3.7 | 4.7 |
| Cr(OH)3 | 4.7 | a |
| Fe(OH)2 | 7.6 | 9.6 |
| Mg(OH)2 | 9.6 | 11.1 |
»ط´ًدآءذ´¦ہي¸ُشü،¢دû³¸ُخغب¾µؤسذ¹طختجâ£؛
(1)½«¸ُشüسأد،ءٍثل½ب،،¢¹آث£¬شع½³ِز؛ضذ¼سبëتتء؟µؤآج·¯(FeSO4،¤7H2O)£¬¼سبëآج·¯µؤؤ؟µؤتا_____________________________________________
(2)شظدٍ½³ِز؛ضذ»؛آ¼سبëةص¼î£¬ضءpH¸ص؛أ´ïµ½4.7£¬¹آث£¬ثùµأ³ءµيµؤ»¯ر§ت½تا____________________________________________________________£»
³£خآدآ£¬Cr(OH)3µؤبـ¶ب»Ksp£½10£32£¬زھت¹Cr3£«حêب«³ءµي[c(Cr3£«)½µضء10£5 mol،¤L£1تسخھ³ءµيحêب«]£¬بـز؛µؤpHس¦µ÷سعa£½________،£
(3)دٍ(2)ضذµأµ½µؤآثز؛ضذ¼سبëءٍثل£¬µ÷½عضء³تا؟ثلذش£¬ثùµأµؤبـز؛تا؛¬____________________بـضتµؤبـز؛،£
(1)½«¸ك¼غسذ¶¾µؤ£«6¼غCr»¹ش³ةخق¶¾µؤ£«3¼غCr
(2)Fe(OH)3،¢Al(OH)3،،5
(3)MgSO4؛حK2SO4
½âخِ

 جىجىدٍةدز»±¾؛أ¾يدµءذ´ً°¸
جىجىدٍةدز»±¾؛أ¾يدµءذ´ً°¸ ذ،ر§ةْ10·ضضسس¦سأجâدµءذ´ً°¸
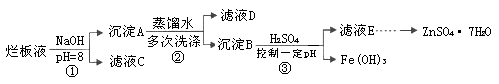
ذ،ر§ةْ10·ضضسس¦سأجâدµءذ´ً°¸ہûسأ·د¾ة¶ئذ؟جْئ¤؟ةضئ±¸´إذشFe3O4½؛جهء£×س¼°¸±²ْخïZnO،£ضئ±¸ء÷³جح¼بçدآ£؛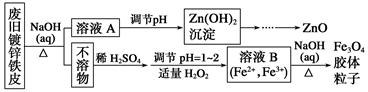
زرضھ£؛Zn¼°ئن»¯؛دخïµؤذشضتسëAl¼°ئن»¯؛دخïµؤذشضتدàثئ£¬اë»ط´ًدآءذختجâ£؛
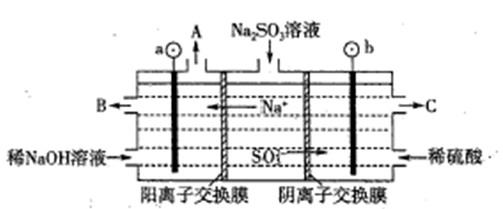
(1)سأNaOHبـز؛´¦ہي·د¾ة¶ئذ؟جْئ¤µؤ×÷سأسذ________،£
| A£®ب¥³سحخغ | B£®بـ½â¶ئذ؟²م | C£®ب¥³جْذâ | D£®¶غ»¯ |
(3)سةبـز؛Bضئب،Fe3O4½؛جهء£×سµؤ¹³جضذ£¬ذë³ضذّح¨بëN2£¬ئنشزٍتا
_______________________________________________________________
(4)Fe3O4½؛جهء£×سؤـ·ٌسأ¼ُر¹¹آث·¨تµدض¹جز؛·ضہë£؟__________(جî،°ؤـ،±»ٍ،°²»ؤـ،±)£¬ہيسةتا________________________________،£
(5)سأضط¸ُثل¼ط·¨(ز»ضضرُ»¯»¹شµخ¶¨·¨)؟ة²â¶¨²ْخïFe3O4ضذµؤ¶¼غجْ؛¬ء؟،£بôذèإنضئإ¨¶بخھ0.010 00 mol،¤L£1µؤK2Cr2O7±ê×¼بـز؛250 mL£¬س¦×¼ب·³ئب،________ g K2Cr2O7(±£ءô4خ»سذذ§ت×ض£¬زرضھMK2Cr2O7£½294.0 g،¤mol£1)،£إنضئ¸أ±ê×¼بـز؛ت±£¬دآءذزائ÷ضذ²»±طزھسأµ½µؤسذ________(سأ±à؛إ±يت¾)،£
¢ظµç×سجىئ½،،¢عةص±،،¢غء؟ح²،،¢ـ²£ء§°ô
¢فبفء؟ئ؟ ¢ق½؛ح·µخ¹ـ،،¢كزئز؛¹ـ
(6) µخ¶¨²ظ×÷ضذ£¬بç¹ûµخ¶¨ا°×°سذK2Cr2O7±ê×¼بـز؛µؤµخ¶¨¹ـ¼â×ى²؟·ضسذئّإف£¬¶ّµخ¶¨½لتّ؛َئّإفدûت§£¬شٍ²â¶¨½ل¹û½«________(جî،°ئ«´َ،±،¢،°ئ«ذ،،±»ٍ،°²»±ن،±)،£
زرضھ25 ،وت±²؟·ضبُµç½âضتµؤµçہëئ½؛â³£تت¾فبç±يثùت¾£؛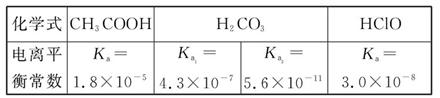
»ط´ًدآءذختجâ£؛
(1)خïضتµؤء؟إ¨¶ب¾ùخھ0.1 mol،¤L-1µؤثؤضضبـز؛£؛
a.CH3COONa b.Na2CO3 c.NaClO d.NaHCO3
pHسةذ،µ½´َإإءذث³ذٍتا (سأ±à؛إجîذ´)،£
(2)³£خآدآ£¬0.1 mol/LµؤCH3COOHبـز؛¼سث®د،تح¹³جضذ£¬دآءذ±ي´ïت½µؤت¾ف±ن´َµؤتا ،£
| A£®c(H+) |
| B£®c(H+)/c(CH3COOH) |
| C£®c(H+)،¤c(OH-) |
| D£®c(OH-)/c(H+) |
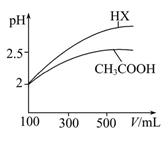
(3)جه»¾ùخھ100 mL pH£½2µؤCH3COOHسëز»شھثلHX£¬¼سث®د،تح¹³جضذpHسëبـز؛جه»µؤ¹طدµبçح¼ثùت¾£¬شٍHXµؤµçہëئ½؛â³£ت (جî،°´َسع،±،¢،°ذ،سع،±»ٍ،°µبسع،±)CH3COOHµؤµçہëئ½؛â³£ت£¬ہيسةتا ،£
دضسذإ¨¶ب¾ùخھ0.1 mol،¤L£1µؤدآءذبـز؛£؛
¢ظءٍثل،¢¢ع´×ثلبـز؛،¢¢غاâرُ»¯ؤئبـز؛،¢¢ـآب»¯ï§بـز؛،¢¢ف´×ثلï§بـز؛،¢¢قءٍثلï§بـز؛،¢¢كءٍثلاâï§بـز؛،¢¢à°±ث®£¬اë»ط´ًدآءذختجâ£؛
£¨1£©¢ظ،¢¢ع،¢¢غ،¢¢ـثؤضضبـز؛ضذسةث®µçہë³ِµؤH£«إ¨¶بسة´َµ½ذ،µؤث³ذٍتا£¨جîذٍ؛إ£©________،£
£¨2£©¢ـ،¢¢ف،¢¢ك،¢¢àثؤضضبـز؛ضذNH4+إ¨¶بسة´َµ½ذ،µؤث³ذٍتا£¨جîذٍ؛إ£©________،£
£¨3£©½«¢غ؛ح¢ـµبجه»»ى؛د؛َ£¬»ى؛دز؛ضذ¸÷ہë×سإ¨¶ب¹طدµصب·µؤتا________،£
| A£®c£¨Na£«£©£½c£¨Cl££©£¾c£¨OH££©£¾c£¨NH4+£© |
| B£®c£¨Na£«£©£½0.1 mol،¤L£1 |
| C£®c£¨Na£«£©£«c£¨NH4+£©£½c£¨Cl££©£«c£¨OH££© |
| D£®c£¨H£«£©£¾c£¨OH££© |
25 ،وت±£¬µçہëئ½؛â³£ت£؛
| »¯ر§ت½ | CH3COOH | H2CO3 | HClO |
| µçہëئ½؛â³£ت | 1.8،ء10£5 | K1 4.3،ء10£7 K2 5.6،ء10£11 | 3.0،ء10£8 |
»ط´ًدآءذختجâ£؛
(1)خïضتµؤء؟إ¨¶بخھ0.1 mol،¤L£1µؤدآءذثؤضضخïضت£؛a.Na2CO3£¬b.NaClO£¬c.CH3COONa£¬d.NaHCO3£»pHسة´َµ½ذ،µؤث³ذٍتا______________(جî±à؛إ)£»
(2)³£خآدآ0.1 mol،¤L£1µؤCH3COOHبـز؛¼سث®د،تح¹³ج£¬دآءذ±ي´ïت½µؤت¾فز»¶¨±نذ،µؤتا________£»،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،،
A£®c(H£«) B.
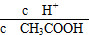 C£®c(H£«)،¤c(OH£) D.
C£®c(H£«)،¤c(OH£) D.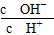
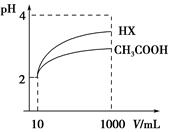
(3)جه»خھ10 mL pH£½2µؤ´×ثلبـز؛سëز»شھثلHXبـز؛·ض±ً¼سث®د،تحضء1 000 mL£¬د،تح¹³جpH±ن»¯بçح¼£؛شٍHXµؤµçہëئ½؛â³£ت________(جî،°´َسع،±،¢،°µبسع،±»ٍ،°ذ،سع،±)´×ثلµؤئ½؛â³£ت£»ہيسةتا__________________________________________£¬د،تح؛َ£¬HXبـز؛ضذث®µçہë³ِہ´µؤc(H£«)________´×ثلبـز؛ث®µçہë³ِہ´c(H£«)(جî،°´َسع،±،¢،°µبسع،±»ٍ،°ذ،سع،±)ہيسةتا£؛____________________________________£»
(4)25 ،وت±£¬CH3COOHسëCH3COONaµؤ»ى؛دبـز؛£¬بô²âµأ»ى؛دز؛pH£½6£¬شٍبـز؛ضذc(CH3COO£)£c(Na£«)£½________(جî×¼ب·تضµ)،£
 ہë×ستؤ؟±ن»¯ا÷تئµؤتا (جî×ضؤ¸)،£
ہë×ستؤ؟±ن»¯ا÷تئµؤتا (جî×ضؤ¸)،£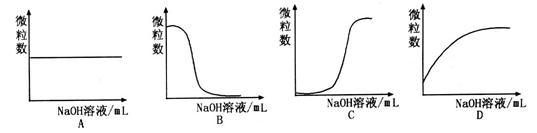
 بـز؛؛ح1جه»0.02mol
بـز؛؛ح1جه»0.02mol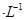 NaOHبـز؛»ى؛د£¬µأµ½2جه»»ى؛دبـز؛،£
NaOHبـز؛»ى؛د£¬µأµ½2جه»»ى؛دبـز؛،£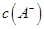 0£®01 mol
0£®01 mol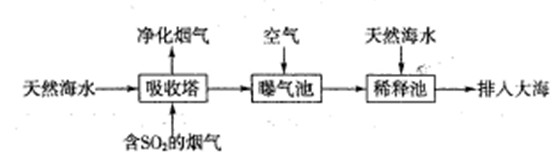
 Fe3+(aq)+3OH-(aq) £» ¦¤H=" a" kJ?mol-1
Fe3+(aq)+3OH-(aq) £» ¦¤H=" a" kJ?mol-1