��Ŀ����
��10�֣���֪��a��H+(aq) + OH-(aq) = H2O(l) ��H=-57.3 kJ?mol-1��
b��1.6gCH4��ȫȼ������ˮ����ʱ����80.2kJ��1gˮ����ת����Һ̬ˮ����2.444kJ��
��1������������������ϡ��Һ������Ӧ��д���������к��ȵ��Ȼ�ѧ����ʽ��
��2��д����������ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����֪2SO2(g)+O2(g) 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
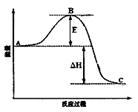
��ͼ��C��ʾ E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ��
�ڸ÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H���D24.8 kJ?mol-1
�� 3Fe2O3(s)+ CO(g)=2Fe3O4(s)+ CO2(g) ��H���D47.2 kJ?mol-1
��Fe3O4(s)+CO(g)=3FeO(s)+CO2(g) ��H�� +640.5 kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
_________________________________________________��
b��1.6gCH4��ȫȼ������ˮ����ʱ����80.2kJ��1gˮ����ת����Һ̬ˮ����2.444kJ��
��1������������������ϡ��Һ������Ӧ��д���������к��ȵ��Ȼ�ѧ����ʽ��
��2��д����������ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����֪2SO2(g)+O2(g)
 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
��ͼ��C��ʾ E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ��
�ڸ÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H���D24.8 kJ?mol-1
�� 3Fe2O3(s)+ CO(g)=2Fe3O4(s)+ CO2(g) ��H���D47.2 kJ?mol-1
��Fe3O4(s)+CO(g)=3FeO(s)+CO2(g) ��H�� +640.5 kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
_________________________________________________��
(10�֣�
(1) NaOH(aq) +1/2H2SO4 (aq) = 1/2Na2SO4(aq) +H2O(l) ��H=-57.3 kJ?mol-1��2�֣�
(2) CH4(g)+2O2(g)=CO2(g)+2 H2O(l) ��H���D889.98 kJ?mol-1��2�֣�
(3) �� ���������� �ޣ���1�֣���2�֣�
�ڽ��� ��Ϊ�����ı��˷�Ӧ������ʹ���E���ͣ���1�֣���2�֣�
(4) FeO(s)+ CO(g)="=" Fe(s)+CO2(g) ��H��-218.0 kJ?mol-1 ��2�֣�
(1) NaOH(aq) +1/2H2SO4 (aq) = 1/2Na2SO4(aq) +H2O(l) ��H=-57.3 kJ?mol-1��2�֣�
(2) CH4(g)+2O2(g)=CO2(g)+2 H2O(l) ��H���D889.98 kJ?mol-1��2�֣�
(3) �� ���������� �ޣ���1�֣���2�֣�
�ڽ��� ��Ϊ�����ı��˷�Ӧ������ʹ���E���ͣ���1�֣���2�֣�
(4) FeO(s)+ CO(g)="=" Fe(s)+CO2(g) ��H��-218.0 kJ?mol-1 ��2�֣�
��1�������Ȼ�ѧ����ʽ����д���к�������һ�������£���ϡ��Һ�У���ͼӦ����1molˮʱ�ų������������Ը÷�Ӧ���Ȼ�ѧ����ʽ�� NaOH(aq) +1/2H2SO4 (aq) = 1/2Na2SO4(aq) +H2O(l) ��H=-57.3 kJ?mol-1��
��2��ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������Ը÷�Ӧ���Ȼ�ѧ����ʽ��CH4(g)+2O2(g)=CO2(g)+2 H2O(l) ��H���D889.98 kJ?mol-1��
��3���ٸ���ͼ���֪��C���ʾ���������������E��ʾ��Ӧ�Ļ�ܣ���ܵĴ�С���ܸı䷴Ӧ�ķ�Ӧ�ȡ�
�ڴ����ı��˷�Ӧ������ʹ���E���ͣ�����B�㽵�͡�
��4�����鷴Ӧ�ȵļ��㡣���ݸ�˹���ɿ�֪�����١�3���ڣ��ۡ�2����6���õ�
FeO(s)+ CO(g)="=" Fe(s)+CO2(g)�����Է�Ӧ���ǡ�H�������D24.8 kJ?mol-1��3��47.2 kJ?mol-1��640.5 kJ?mol-1��2������218.0 kJ?mol-1��
��2��ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������Ը÷�Ӧ���Ȼ�ѧ����ʽ��CH4(g)+2O2(g)=CO2(g)+2 H2O(l) ��H���D889.98 kJ?mol-1��
��3���ٸ���ͼ���֪��C���ʾ���������������E��ʾ��Ӧ�Ļ�ܣ���ܵĴ�С���ܸı䷴Ӧ�ķ�Ӧ�ȡ�
�ڴ����ı��˷�Ӧ������ʹ���E���ͣ�����B�㽵�͡�
��4�����鷴Ӧ�ȵļ��㡣���ݸ�˹���ɿ�֪�����١�3���ڣ��ۡ�2����6���õ�
FeO(s)+ CO(g)="=" Fe(s)+CO2(g)�����Է�Ӧ���ǡ�H�������D24.8 kJ?mol-1��3��47.2 kJ?mol-1��640.5 kJ?mol-1��2������218.0 kJ?mol-1��

��ϰ��ϵ�д�
�����Ŀ

 =
=


 ��
��
 ȼ���ȵĻ�ѧ����ʽΪ��
ȼ���ȵĻ�ѧ����ʽΪ��




 ��2H2O��g�� ��H2����483.6 kJ��
��2H2O��g�� ��H2����483.6 kJ��
 �Ħ�HΪ____________________��
�Ħ�HΪ____________________��