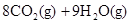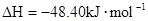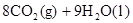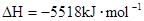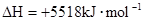��Ŀ����
(12��) �״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ����
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦI�� CO(g) �� 2H2(g) CH3OH(g) ��H1
CH3OH(g) ��H1
��ӦII�� CO2(g) �� 3H2(g) CH3OH(g) + H2O(g) ��H2
CH3OH(g) + H2O(g) ��H2
��������Ӧ���ϡ�ԭ�Ӿ��á�ԭ����� ���I������
����֪��Ӧ��������仯��ͼ��ʾ���ɱ��������ж� ��H1 0 �����������������������

��ij�¶��£���2 mol CO��6 mol H2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)�� 0.2 mol��L�� ��CO��ת����Ϊ
��2����֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(g) ��H ����1275.6 kJ��mol
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H ����566.0 kJ��mol
�� H2O(g) �� H2O(l) ��H ����44.0 kJ��mol
�����1 mol�״�����ȫȼ������1 molһ����̼��Һ̬ˮ�ų�������Ϊ________
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ�����������ͼ��ʾ�ĵ��װ�á�

�ٸõ�������ĵ缫��ӦΪ____________
�ڹ���һ��ʱ������Һ��pH��С���õ���ܷ�Ӧ�Ļ�ѧ����ʽΪ____________.
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦI�� CO(g) �� 2H2(g)
 CH3OH(g) ��H1
CH3OH(g) ��H1��ӦII�� CO2(g) �� 3H2(g)
 CH3OH(g) + H2O(g) ��H2
CH3OH(g) + H2O(g) ��H2��������Ӧ���ϡ�ԭ�Ӿ��á�ԭ����� ���I������
����֪��Ӧ��������仯��ͼ��ʾ���ɱ��������ж� ��H1 0 �����������������������

��ij�¶��£���2 mol CO��6 mol H2����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO)�� 0.2 mol��L�� ��CO��ת����Ϊ
��2����֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) �� 2CO2(g) �� 4H2O(g) ��H ����1275.6 kJ��mol
�� 2CO (g)+ O2(g) �� 2CO2(g) ��H ����566.0 kJ��mol
�� H2O(g) �� H2O(l) ��H ����44.0 kJ��mol
�����1 mol�״�����ȫȼ������1 molһ����̼��Һ̬ˮ�ų�������Ϊ________
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ�����������ͼ��ʾ�ĵ��װ�á�

�ٸõ�������ĵ缫��ӦΪ____________
�ڹ���һ��ʱ������Һ��pH��С���õ���ܷ�Ӧ�Ļ�ѧ����ʽΪ____________.
��1����1 �ڣ� ��80% ��2��442.8KJ
��3����O2+2H2O+4e-=4OH- ��2CH3OH+3O2+4OH-=2CO32-+6H2O
��3����O2+2H2O+4e-=4OH- ��2CH3OH+3O2+4OH-=2CO32-+6H2O
��1���ٸ��ݷ���ʽ��֪����ӦI�з�Ӧ��ȫ��ת��Ϊ�״������ϡ�ԭ�Ӿ��á�ԭ��
�ڸ���ͼ���֪����Ӧ�������������������������������Է�Ӧ�Ƿ��ȷ�Ӧ����H1��0��
�۷�ӦǰCO��Ũ����1mol/L���������ĵ�CO��1mol/L��0.2mol/L��0.8mol/L��������ת������80����
��2�����鷴Ӧ�ȵ��йؼ��㡣���ݸ�˹���ɿ�֪�����٣��ڣ��ۡ�4����2���õ�CH3OH(l) �� O2(g) �� CO2(g) �� 2H2O(l)�����Է�Ӧ���ǡ�H������1275.6 kJ��mol��566.0 kJ��mol��44.0 kJ��mol��4����2����442.8kJ��mol������1 mol�״�����ȫȼ������1 molһ����̼��Һ̬ˮ�ų�������Ϊ442.8kJ��
��3����ԭ����������õ����ӣ���������������ͨ�롣���ڵ����������������Һ������������Ӧʽ��O2+2H2O+4e-=4OH-��
�����ڸ����Ǽ״�ʧȥ���ӣ�����̼��غ�ˮ�������ܷ�Ӧʽ��2CH3OH+3O2+4OH-=2CO32-+6H2O��
�ڸ���ͼ���֪����Ӧ�������������������������������Է�Ӧ�Ƿ��ȷ�Ӧ����H1��0��
�۷�ӦǰCO��Ũ����1mol/L���������ĵ�CO��1mol/L��0.2mol/L��0.8mol/L��������ת������80����
��2�����鷴Ӧ�ȵ��йؼ��㡣���ݸ�˹���ɿ�֪�����٣��ڣ��ۡ�4����2���õ�CH3OH(l) �� O2(g) �� CO2(g) �� 2H2O(l)�����Է�Ӧ���ǡ�H������1275.6 kJ��mol��566.0 kJ��mol��44.0 kJ��mol��4����2����442.8kJ��mol������1 mol�״�����ȫȼ������1 molһ����̼��Һ̬ˮ�ų�������Ϊ442.8kJ��
��3����ԭ����������õ����ӣ���������������ͨ�롣���ڵ����������������Һ������������Ӧʽ��O2+2H2O+4e-=4OH-��
�����ڸ����Ǽ״�ʧȥ���ӣ�����̼��غ�ˮ�������ܷ�Ӧʽ��2CH3OH+3O2+4OH-=2CO32-+6H2O��

��ϰ��ϵ�д�
�����Ŀ
 2NH3(g), ��H="-92.4" kJ��mol��1,��H��H���ļ����ǣ���������
2NH3(g), ��H="-92.4" kJ��mol��1,��H��H���ļ����ǣ��������� ===H2O��g�� ��H1��a kJ��
===H2O��g�� ��H1��a kJ��
 ===2H2O��g�� ��H2��b kJ��
===2H2O��g�� ��H2��b kJ�� ===2H2O��l�� ��H4��d kJ��
===2H2O��l�� ��H4��d kJ�� �����飩ȼ�����ɶ�����̼��Һ̬ˮʱ�ų�48.40kJ��������ʾ������Ӧ���Ȼ�ѧ����ʽ��ȷ���ǣ�����
�����飩ȼ�����ɶ�����̼��Һ̬ˮʱ�ų�48.40kJ��������ʾ������Ӧ���Ȼ�ѧ����ʽ��ȷ���ǣ�����