��Ŀ����
10�������ҹ������ƾõ���ʷ�����dz�˵�ġ���������£��������ν��ײ衱�о��дף�˵���˴������������е���Ҫ�ԣ���1������ԭʼ���ĩ�ڣ��ҹ������ѧ�����ù�����ƣ����Ǿ��ڿ����г��ڷ��þͻ��Ϊ�����ơ�����ƣ�����д�����������ơ�����Ļ�ѧ����ʽCH3CH2OH+O2��CH3COOH+H2O��
��2�������ܽ�ʳƷ�к��ơ��ס�����Ԫ�ص����ʣ��������������Ԫ�ص����գ�д������ĵ��뷽��ʽ��CH3COOH
 CH3COO-+H+��
CH3COO-+H+����3�������ճ������ж������ã����ô���ϴȥ������ͭ��������Ʒ������⼣�����ˮ���е�ˮ��������ˮ������Ҫ�ɷ���CaCO3����ϴ������Ѭ�������պͺ��յȣ�д�������ˮ�������ӷ���ʽCaCO3+2CH3COOH=Ca2++2CH3COO-+H2O+CO2����
��4������ʱ�ȼӴ��ּӾƿ������Ӳ˵���ζ����ԭ����������֬�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽCH3COOH+CH3CH2OH?CH3COOCH2CH3+H2O���÷�Ӧ��������������Ӧ��
���� ��1���Ҵ��������������ᣬ���������ζ��
��2��ˮ������Ҫ�ɷ�Ϊ̼��ƣ�������̼��Ʒ�Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2CH3COOH=��CH3COO��2Ca+H2O+CO2�������ݻ�ѧ����ʽд����ȷ�����ӷ���ʽ��
��3�����ݴ�����һԪ���ᣬ���ֵ��룻
��4������Ҫ�ɷ�ΪCH3COOH���Ƶ���Ҫ�ɷ�ΪCH3CH2OH�����߿��Է���������Ӧ����CH3COOCH2CH3��
��� �⣺��1���Ҵ��������������ᣬ��Ӧ�ķ���ʽΪ��CH3CH2OH+O2��CH3COOH+H2O��
�ʴ�Ϊ��CH3CH2OH+O2��CH3COOH+H2O��
��2��������̼��Ʒ�Ӧ���ɴ���ƺ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��CaCO3+2CH3COOH=��CH3COO��2Ca+H2O+CO2������Ӧ�����ӷ���ʽΪ��CaCO3+2CH3COOH=Ca2++2CH3COO-+H2O+CO2����
�ʴ�Ϊ��CaCO3+2CH3COOH=Ca2++2CH3COO-+H2O+CO2����
��3��������һԪ���ᣬ���ֵ��룬���뷽��ʽΪ��CH3COOH CH3COO-+H+��
CH3COO-+H+��
�ʴ�Ϊ��CH3COOH CH3COO-+H+��
CH3COO-+H+��
��4������Ҫ�ɷ�ΪCH3COOH���Ƶ���Ҫ�ɷ�ΪCH3CH2OH�����߿��Է���������Ӧ����CH3COOCH2CH3����ѧ����ʽΪ��CH3COOH+CH3CH2OH?CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH?CH3COOCH2CH3+H2O��������Ӧ��
���� ���⿼���Ҵ�����������ʣ��漰�Ҵ��Ĵ�������Ӧ�Լ������ᷢ����������Ӧ��֪ʶ�������л���Ӧ�Ķ��ѻ�ѧ�����γɻ�ѧ�����������Ӧ�������ǽ���Ĺؼ���

| A�� | �������Ƶĵ���ʽ��Na${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$Na | |
| B�� | ������Ϊ35��������Ϊ45����ԭ�ӣ�${\;}_{35}^{80}$Br | |
| C�� | �����ӵĽṹʾ��ͼ��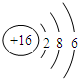 | |
| D�� | ����ױ��Ľṹ��ʽ�� |
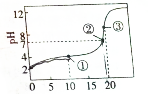 25��ʱ����20mL0.2mo•lL-1HR��Һ������0.2mol•L-1NaOH��Һ���õ���ͼ�ĵζ����ߣ���������Ũ�ȵĹ�ϵʽ������ǣ�������
25��ʱ����20mL0.2mo•lL-1HR��Һ������0.2mol•L-1NaOH��Һ���õ���ͼ�ĵζ����ߣ���������Ũ�ȵĹ�ϵʽ������ǣ�������| A�� | �����Һ�У�c��HR��+2c��H+��=c��R-��+2c��OH-�� | |
| B�� | �����Һ�У�c��Na+��=c��HR��+c��R-�� | |
| C�� | �����Һ�У�c��Na+����c��R-������OH-����c��H+�� | |
| D�� | �ζ������п��ܻ���֣�c��HR����c��R-������H+����c��Na+�� |
| A�� | 950mL��76g | B�� | 500mL��80g | C�� | 1000mL��80g | D�� | 1000mL��76g |
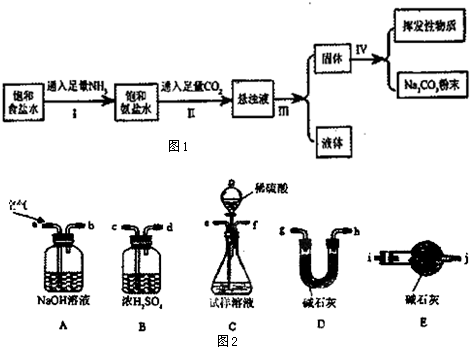
�ڳ����£��й����ʵ��ܽ��Ϊ��
| ���� | NH4Cl | NaHCO3 | Na2CO3 | NaCl |
| �ܽ��/g | 37.2 | 9.6 | 21.5 | 36.0 |
��2������I��II�ܷ�Ӧ�����ӷ���ʽΪNa++NH3+CO2+H2O�TNaHCO3��+NH4+��
��3������I��II���ܵߵ���ԭ������NH3�ڱ���NaCl��Һ���ܽ�����CO2����ͨNH3����Һ�ʼ��ԣ���������CO2������NaHCO3�����ɣ�����Ӧ��ͨ��NH3��
��4���������õ�̼���Ʒ�ĩ�Ƿ���NaHCO3����ʵ�鷽���ǣ�д���������衢�����ۣ���ȡ�����������Թܣ����ȣ�������������ͨ�����ʯ��ˮ����ʹ����ʯ��ˮ����ǣ�֤����NaHCO3��
��5��Ϊ�˲ⶨ����ȡ����Ĵ��ȣ���������ֻ��̼�����ƣ�����С���ʵ�鲽��Ϊ��
i��ʹ������װ����װʵ��װ�ã������������
ii����ȡWg��Ʒ����Cװ�õ���ƿ�У�����������ˮ�ܽ�
iii������Dװ�õ�����ΪW1 g
iv���ӷ�Һ©������ϡ���ᣬֱ�����ٲ�������Ϊֹ
v����a����������һ�����Ŀ������ٴγ���Dװ������ΪW2 g
vi���ظ�����v�IJ�����ֱ��Dװ�õ��������ٸı䣬�Ƶ�Dװ�õ�����ΪW3 g
��������ʵ��ش��������⣺
�ٵ�i����ʹ������װ�����ӵĽӿ�˳��Ϊ����b������e����f������c����d������g����h��[��h����g��]����i����
�ڵڶ���ʢ��ʯ��װ�õ������Ƿ�ֹ��������D�м�ʯ�Ҹ���ʵ�飮
�۲�����̼���ƺ�̼�����Ƶ����ʵ���֮��Ϊ$\frac{{w}_{3}-{w}_{1}}{44}$mol��