��Ŀ����
����Ŀ��A��B��C��D��EΪǰ������Ԫ�أ���ԭ������������������AB�����������ɫ��Cԭ���ڲ��������������������5����D��ͬ����Ԫ���е縺�����E����������Һ����ɫ���ش��������⣺
��1��Aԭ����________��������ͬ�ĵ��ӣ�Dԭ�ӵ�M�ܲ���ӵ��˶�״̬��________�֡�
��2��Ԫ��A�ĵ�һ������________������ڡ���С�ڡ����ڡ���Ԫ��B�ĵ�һ�����ܣ�AB2-�����幹����________������AB�к��е���������������֮��Ϊ________��
��3��Ԫ��C��D���γɵĻ������ij�־����ṹ��ͼ��ʾ�������������������ӵĸ�������________��
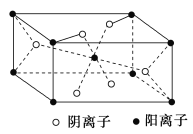
��4��E��̬ԭ�ӵĺ�������Ų�ʽΪ________����E���ʵķ�ĩ����A�ļ���̬�⻯���Ũ��Һ�У������Ϲ��������ַ�Ӧ���õ�����ɫ����Һ���÷�Ӧ�����ӷ���ʽ��________��
���𰸡�3 7 ���� V�� 1��2 2��1 1s22s22p63s23p63d104s1��[Ar]3d104s1 2Cu+8NH3��H2O+O2=2[Cu(NH3)4]2��+4OH-+6H2O
��������
A��B��C��D��EΪǰ������Ԫ�أ���ԭ��������������������AB�����������ɫ��֪��AΪNԪ�ء�BΪOԪ�أ�Cԭ���ڲ��������������������5������C��MgԪ�أ�E����������Һ����ɫ����EΪCuԪ�أ�D��ԭ������С��E������ͬ����Ԫ���е縺�������DΪClԪ�ء�
��1��AΪNԪ�أ�ԭ�ӵĵ����Ų�ʽΪ1s22s22p4����ԭ�Ӻ�����1s��2s��2p����������ͬ�ĵ��ӣ�DΪClԪ�أ�M�ܲ������߸����ӣ�����ӵ��˶�״̬�����֣��ʴ�Ϊ3��7��
��2��Nԭ��2p���Ϊ��������ȶ�״̬����Ԫ��N�ĵ�һ�����ܴ���Ԫ��O�ĵ�һ�����ܣ�NO2-�е�ԭ�ӵŵ��Ӷ���Ϊ![]() ���۲���Ӷ���Ϊ
���۲���Ӷ���Ϊ![]() ����NO2-�����幹����V�Σ�NOΪֱ���ͼ��Է��ӣ�������Nԭ�Ӳ�ȡsp�ӻ�����1��������1��������һ��������������������������֮��Ϊ1:2���ʴ�ΪС�ڣ�V�� ��1��2��
����NO2-�����幹����V�Σ�NOΪֱ���ͼ��Է��ӣ�������Nԭ�Ӳ�ȡsp�ӻ�����1��������1��������һ��������������������������֮��Ϊ1:2���ʴ�ΪС�ڣ�V�� ��1��2��
��3�������������Ӵ��������붥�㣬��������������ĿΪ![]() �������Ӵ������ڼ����ϣ���������������ĿΪ
�������Ӵ������ڼ����ϣ���������������ĿΪ![]() ���ʾ������������������ӵĸ�������4��2=2��1���ʴ�Ϊ2:1��
���ʾ������������������ӵĸ�������4��2=2��1���ʴ�Ϊ2:1��
��4����Cu���ʵķ�ĩ����NH3��Ũ��Һ�У�ͨ��O2��ͭ���������������İ���ͭ�����ӣ���Ӧ�����ӷ���ʽΪ2Cu+8NH3��H2O+O2=2[Cu(NH3)4]2��+4OH-+6H2O���ʴ�Ϊ2Cu+8NH3��H2O+O2=2[Cu(NH3)4]2��+4OH-+6H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������������ȡ��ˮ�����ʡ����ᡢ��Ρ�����ȣ���˱��㷺Ӧ���ڻ������Ṥ�����ʡ���ҩ���ϳ���ά�����ϵ���ҵ��
��1���Լ���Ϊԭ�Ͽ��Ƶúϳɰ��õ��������йط�Ӧ�������仯����ͼ��ʾ��
 ��
��

д��CH4(g)��H2O(g)��Ӧ����CO(g)��H2(g)���Ȼ�ѧ����ʽ��_______________��
��2����֪N2(g)��3H2(g)![]() 2NH3(g)����H= -94.4kJ��mol-1����ij�ݻ�Ϊ2L�ĺ��������з����ϳɰ���Ӧ����ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ��
2NH3(g)����H= -94.4kJ��mol-1����ij�ݻ�Ϊ2L�ĺ��������з����ϳɰ���Ӧ����ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ��

��ǰ20min��v(NH3)=________mol/(L��min)���ų�������Ϊ________��
��45minʱ��ȡ�Ĵ�ʩ��________��ʱ��III�����·�Ӧ��ƽ�ⳣ��Ϊ________������3λ��Ч���֣���
��3��һ���¶��£�����ͬ����CO2��NH3�ֱ�ͨ��ij�����ܱ������н������з�Ӧ��2NH3(g)+CO2(g)=CO(NH2)2(l)+H2O(g)����H��0���õ��������ݣ�
ʵ���� | ��ʼ��/mol | ƽ��ʱ��ת���� | ƽ��ʱ����ƽ����Է������� | ||
NH3(g) | CO2(g) | NH3(g) | CO2(g) | ||
1 | 2 | 1 | a1 | a2 | M1 |
2 | 4 | 2 | a3 | a4 | M2 |
3 | n1 | n2 | a5 | a6 | M3 |
��a1________a4������������������������������M1________ M2������������������������������
����a5>a6����n1��n2��������Ĺ�ϵ��________��
��4������ʱ����ͨ��500mL 0.1mol��L-1��������pH=6��������Һ������Ũ���ɴ�С��˳����____________________________________________��
��5������������KOH��Һ�ɹ���ȼ�ϵ�أ���Ӧԭ��Ϊ4NH3+3O2=2N2+6H2O����Ӧһ��ʱ��������Һ��pH��________����������������С����������������
����Ŀ��Ŀǰ��ҵ�Ͽ�����CO��CO2������ȼ�ϼ״���ij�о�С��������йؼ״���ȡ��������ѧ��Ӧԭ������̽������֪�ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ��(K1��K2��K3)���±���ʾ��
��ѧ��Ӧ | �ʱ� | ƽ�ⳣ�� | �¶�/�� | ||
500 | 700 | 800 | |||
��2H2(g)��CO(g) | ��H1 | K1 | 2.5 | 0.34 | 0.15 |
��CO2(g)��H2(g) | ��H2 | K2 | 1.0 | 1.70 | 2.52 |
��CO2(g)��3H2(g) | ��H3 | K3 | |||
��ش��������⣺
(1)��Ӧ����________(��������������������)��Ӧ��
(2)���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��________(��K1��K2��ʾ)�����ݷ�Ӧ���ж���S___0(����>��������������<��)����______(�����ϸ��������ϵ���)�¶��������ڸ÷�Ӧ�Է����С�
(3)500��ʱ����÷�Ӧ����ijʱ�̣�CO2(g)��H2(g)��CH3OH(g)��H2O(g)��Ũ�ȷֱ�Ϊ0.1mol/L��0.8mol/L��0.3mo/L��0.15mol/L�����ʱv��________v��(����>��������������<��)��







