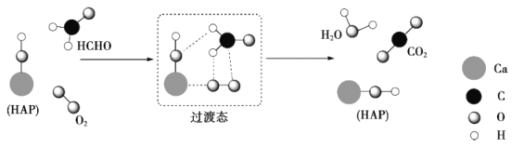
��Ŀ����
����Ŀ����ͼ��������ʯī���缫����ⱥ��Na2SO4��Һ��װ�ã�U�ι��ڵĵ��Һ�����ȼ��з�ָ̪ʾ�����ش��������⣺
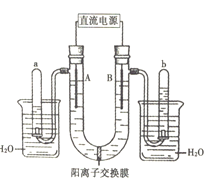
(1)����ͼʾ�е����Թ��ڵ�Һ��߶ȣ��ж�ֱ����Դ���Ϊ______����Na+ͨ�������ӽ���Ĥ�ƶ��ķ���Ϊ______(������������������������)��
(2)�������У��йص缫������Һ��ɫ�仯����ȷ����______(�����)��
��A����Һ����ɫ��Ϊ��ɫ
��B����Һ����ɫ��Ϊ��ɫ
��A����Һ����ɫ
��B����Һ����ɫ
(3)д��B���з����ĵ缫��Ӧ______��
(4)����b��������ķ�����������______��
���𰸡��� ������ �٢� 2H2O-4e-=O2��+4H+ ��Ĵָ��ס�Թܿڣ�ȡ���Թܣ��������ƿ�Ĵָ���������ǵ�ľ�������Թ��ڣ������ǵ�ľ����ȼ
��������
������ʯī���缫��ⱥ��Na2SO4��Һ��ʵ���ǵ��ˮ������a��b�����Թ��ڵ�Һ��߶ȿ�֪��a�Թ��ռ���������������b�Թ��ռ�����������������AΪ��������BΪ�������������缫��ӦʽΪ2H2O+2e-=H2��+2OH-�������缫��ӦʽΪ2H2O-4e-=O2��+4H+���ݴ˷������
(1)��ʯī�缫��ⱥ��Na2SO4��Һʵ���ǵ��ˮ������ͼʾ�е����Թ��ڵ�Һ��߶ȿ�֪��a�Թ��ռ���������������b�Թ��ռ�����������������AΪ��������BΪ���������������Դ������ӡ��������Դ������ӣ�����ֱ����Դ���Ϊ���������ʱ���������Һ��������ͨ�������ӽ���Ĥ����������A����Na+ͨ�������ӽ���Ĥ���ƶ����������ʴ�Ϊ������������
(2)�����ĵ缫��ӦʽΪ2H2O+2e-=H2��+2OH-�����ɵ�OH-ʹ��������̪��죬�����ĵ缫��ӦʽΪ2H2O-4e-=O2��+4H+������H+����������̪����ɫ����A����Һ����ɫ��Ϊ��ɫ��B����Һ����ɫ���ʴ�Ϊ���٢ܣ�
(3)�õ�����AΪ��������BΪ������������B���з����ĵ缫��ӦΪ2H2O-4e-=O2��+4H+���ʴ�Ϊ��2H2O-4e-=O2��+4H+��
(4)������AΪ��������BΪ��������b��������Ϊ���������ô����ǵ�ľ�����飬����Ϊ����Ĵָ��ס�Թܿڣ�ȡ���Թܣ��������ƿ�Ĵָ���������ǵ�ľ�������Թ��ڣ������ǵ�ľ����ȼ���ʴ�Ϊ����Ĵָ��ס�Թܿڣ�ȡ���Թܣ��������ƿ�Ĵָ���������ǵ�ľ�������Թ��ڣ������ǵ�ľ����ȼ��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�����Ŀ��ij�¶��£���4.0 L�����ܱ������г���2.0 mol PCl5����ӦPCl5(g) ![]() PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
ʱ��/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3)/mol | 0 | 0.32 | 0.38 | 0.40 | 0.40 |
����˵����ȷ����(����)
A.��Ӧ��ǰ50 s��ƽ����Ӧ����Ϊv(PCl3)��0.006 4 mol/(L��s)
B.��ͬ�¶��£���ʼʱ��������г���4.0 mol PCl3��4.0 mol Cl2���ﵽƽ��ʱ��PCl3��ת����С��80%
C.��ͬ�¶��£���ʼʱ��������г���2.0 mol PCl5��0.4 mol PCl3��0.40 mol Cl2���ﵽƽ��ǰv(��)>v(��)
D.���������������䣬�����¶ȣ�ƽ��ʱ��c(PCl3)��0.11 mol/L����Ӧ����H<0
����Ŀ����������������ұ�����Ӽ���ռ�������������������� 80%���ϣ�����������л������Ĵ�����Ϊ������V2O5 �������ʣ����ǴӷϷ���������Ҫ�ɷ�V2O5��VOSO4��K2SO4��SiO2 ��Fe2O3 �ȣ� �л��� V2O5 ��һ��������������ʾ��ͼ��
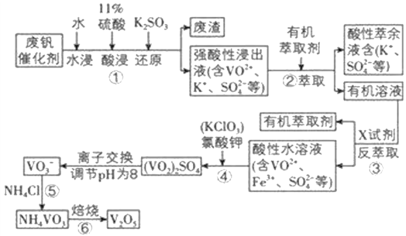
���ֺ���������ˮ�е��ܽ��������ʾ���ش��������⣺
���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
��1�����з�������Ҫ�ɷ��� __________________________������V2O5 ������Ӧ�����ӷ���ʽΪ ___________________________ ��
��2���ڡ����еı仯���̿ɼ�Ϊ����ʽ�е�R ��ʾ VO2+�� Fe3+��HA ��ʾ�л���ȡ������Ҫ�ɷ֣�R2(SO4)n��ˮ�㣩+2nHA���л��㣩 2RA���л��㣩+ nH2SO4��ˮ�㣩��������ȡʱ��������������ԭ���� ______________________��ʵ���ҽ�����ȡ����ʹ�õIJ�������Ϊ ______________________��
��3��ʵ�����õ�ԭ����V2O5 ռ 6%��ԭ���е����з��ѻ���� V2O5����ȡ 100 g �÷Ϸ���������ҵ�����IJ������ʵ�飬������ 100 mL 0.1 molL -1 ��KClO3 ��Һʱ����Һ�еķ�ǡ�ñ���ȫ�����������Ժ������û����ʧ�����ʵ���з��Ļ������� __________________[M(V2O5)��182 gmol -1]��
��4��25��ʱ��ȡ������ʵ��������õ��������ʺ���Һ pH ֮��Ĺ�ϵ�����ʾ��
pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 |
��������/% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.6 | 96.4 | 93.1 |
���ж���ʵ������ʱ�����м��� NH4Cl ������Һ����� pHΪ____________������������Ϊ 93.1%ʱ������ Fe(OH)3 ���������ʱ��Һ�� c(Fe3+)��____________ ����֪��25��ʱKsp[Fe(OH)3]��2.6��10 -39����