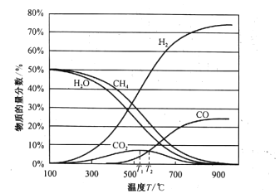
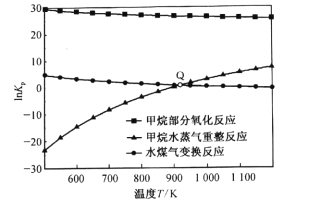
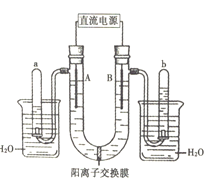
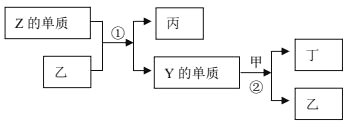
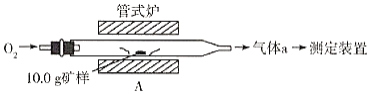
��Ŀ����
����Ŀ���������һ����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͽ�ͨ������;���Ʊ���

��ش��������⣺
��1�������ϩ�����������__��ԭ�ӹ�ƽ�档
��2�������ķ���ʽΪ__������ȴ�����_�֡�
��3����֪ϩ���ܷ������·�Ӧ��
 ��
��
��д�����з�Ӧ����Ľṹ��ʽ��![]()
![]() __��
__��
��4��A�Ƕ��ۻ����ϩ��ͬ���칹�壬��ʹ������Ȼ�̼��Һ��ɫ��A���������������Һ�����������Եõ��Ա���������ʾ�������ϵ����(��CH3����CH2R����CHR2)��ϩ���������������������Һ�������Ȼ���д��A���п��ܵĽṹ��ʽ�����������칹��__��
���𰸡�9 C10H16 6 OHC-CHO+OCHCH2CHO ![]() ��
��![]() ��
��![]()
��������
��1����˫��ֱ̼��������ԭ��һ����ͬһƽ���ڣ�
��2�����ݽṹ��ʽ�ж��л���ķ���ʽ�ͻ�����Ŀ��
��3������Ϣ��֪��̼̼˫����̼������Ϊȩ����
��4����ʹ������Ȼ�̼��Һ��ɫ��Ӧ����̼̼˫����A���������������Һ�����������Եõ��Ա������ᣬ˵������λ�ڶ�λλ�ã�
![]() ̼̼˫��Ϊƽ��ṹ��̼̼˫���γ�2��ƽ����Թ��棬��˫��ֱ̼��������ԭ��һ����ͬһƽ���ڣ���5��C��4��H��ͬһƽ���ڣ�
̼̼˫��Ϊƽ��ṹ��̼̼˫���γ�2��ƽ����Թ��棬��˫��ֱ̼��������ԭ��һ����ͬһƽ���ڣ���5��C��4��H��ͬһƽ���ڣ�
�ʴ�Ϊ��9��
![]() �ɽ����ṹ��ʽ����֪�����ķ���ʽΪ
�ɽ����ṹ��ʽ����֪�����ķ���ʽΪ![]() ����������ֻ�дμ����Ǽ�������H�����Ը����ʵ�һ�ȴ�����2�֣����μ���һ����ԭ��ȡ�����ȴ�����3�֣����Ǽ���һ����ԭ��ȡ�����ȴ�����3�֣���6�֣�
����������ֻ�дμ����Ǽ�������H�����Ը����ʵ�һ�ȴ�����2�֣����μ���һ����ԭ��ȡ�����ȴ�����3�֣����Ǽ���һ����ԭ��ȡ�����ȴ�����3�֣���6�֣�
�ʴ�Ϊ��![]() ��6��
��6��
![]() ����Ϣ��֪��̼̼˫����������������Ӧ����������Ϊ̼̼˫�����ѣ����ѵ�̼ԭ��������ԭ���γ��µ��л��������
����Ϣ��֪��̼̼˫����������������Ӧ����������Ϊ̼̼˫�����ѣ����ѵ�̼ԭ��������ԭ���γ��µ��л��������![]()
![]()
![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��
![]() ���ۻ����ϩ�ķ���ʽΪ
���ۻ����ϩ�ķ���ʽΪ![]() ��A�Ƕ��ۻ����ϩ��ͬ���칹�壬��A�ķ���ʽΪ
��A�Ƕ��ۻ����ϩ��ͬ���칹�壬��A�ķ���ʽΪ![]() �������Ͷ�Ϊ5��A�����Ը��������Һ�����������Եõ��Ա������ᣬ��A��ʹ������Ȼ�̼��Һ��ɫ��˵����������
�������Ͷ�Ϊ5��A�����Ը��������Һ�����������Եõ��Ա������ᣬ��A��ʹ������Ȼ�̼��Һ��ɫ��˵����������![]() ������������λȡ�����������ӱ�����̼ԭ���Ϻ�����ԭ�ӣ�A���ܵĽṹ��ʽ�У�
������������λȡ�����������ӱ�����̼ԭ���Ϻ�����ԭ�ӣ�A���ܵĽṹ��ʽ�У� ��
��
�ʴ�Ϊ�� ��
��