��Ŀ����
20��Ϊ��̽��AgNO3�����ȶ��Ժ������ԣ�ij��ѧ��ȤС�����������ʵ�飮��AgNO3�����ȶ���
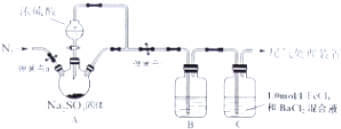
������ͼ��ʾ��ʵ��װ��A����AgNO3���壬��������ɫ���壬��װ��D���ռ�����ɫ���壮����Ӧ�������Թ��в�������Ϊ��ɫAg�����гּ�����������ʡ�ԣ�
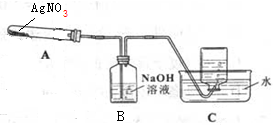
��1����μ���װ�õ������ԣ����Ӻ���������δ��ҩƷ֮ǰ�����Թܣ����C�е�����������ð����˵�����������ã�
��2��C�м���ƿ�ռ�������ΪO2��Ҫ�ռ������ĸ����壬��ȷ�IJ����ǵȵ������������ݾ��ȷų�ʱ���ռ���
��3��AgNO3�ֽ�Ļ�ѧ����ʽΪ2AgNO3$\frac{\underline{\;\;��\;\;}}{\;}$2Ag+2NO2��+O2����
��4����ͬѧ��Ϊ��ƾ�Թ�A�еĺ���ɫ������������ж�NO2�����Ǵ���ģ���Ϊ��ɫNO��������Ҳ���ɺ���ɫ�����Լ���ǰӦ���ž�װ���еĿ��������Ƿ�ͬ�Ĺ۵㣿��˵�����ɷ��������ֽ�����������
��AgNO3��������
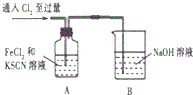
����������˿����AgNO3��Һ�Ĵ��Թ��У�һ��ʱ�����˿ȡ����Ϊ������Һ��Fe����������²����£�
����һ��Fe��������Fe3+��
�������Fe��������Fe2+��
��������Fe��������Fe3+��Fe2+��
��ѡ�õ��Լ���KSCN��Һ������KMnO4��Һ����ˮ�����ᣬ���ᣬNaCl��Һ
������±���
| ���� | ���� | ���ۻ�Ŀ�� |
| ��1��ȡ������Һ���Թ��У�����Һ�м�������NaCl��Һ | - | ����Ag+ |
| ��2��ȡ����������Һ���Թ��м���KSCN��Һ���� | ��Һ��Ѫ��ɫ | ����Fe3+ |
| ��3��ȡ��1��������������Һ���Թ��У���������KMnO4��Һ���� | ��ɫ��ʧ | ����Fe2+ |
���� I����1�����Ӻ���������δ��ҩƷ֮ǰ�����Թܣ����C�е�����������ð����˵�����������ã�
�ʴ�Ϊ�����Ӻ���������δ��ҩƷ֮ǰ�����Թܣ����C�е�����������ð����˵�����������ã�
��2����������������ʱ���к���ɫ�������ɣ��������Ƕ�����������Ag���ɣ�����Ԫ�ػ��ϼ۱仯֪�����л��ϼ����ߵ��������ɣ�ֻ�������������Ը÷�Ӧ����ʽΪ2AgNO3$\frac{\underline{\;\;��\;\;}}{\;}$2Ag+2NO2��+O2�������������ͨ��NaOH��Һʱ��������Ӧ4NO2+O2+2H2O=4HNO3�����ݸ÷�Ӧ����ʽ2AgNO3$\frac{\underline{\;\;��\;\;}}{\;}$2Ag+2NO2��+O2����4NO2+O2+2H2O=4HNO3֪��������ʣ�࣬����C���ռ���������O2��Ҫ�ռ������ĸ����壬Ӧ�õȵ������������ݾ��ȷų�ʱ�ռ���
��3�����ݣ�2���ķ�����д����ʽΪ��
��4���������ֽ�����в��������������ܺ�NO��Ӧ�ģ�
II���������Fe������ΪFe2+��
��1���ڼ���Fe3+��Fe2+ǰӦ�ó�ȥAg+�ĸ��ţ�
��2��Fe3+��KSCN��Һ����������ʹ��Һ��Ѫ��ɫ��
��3��Fe2+���л�ԭ�ԣ��ܱ�ǿ���������Ը��������Һ������ʹ���Ը��������Һ��ɫ��
��� �⣺I����1������ѹǿ�����װ�������ԣ�
��2����������������ʱ���к���ɫ�������ɣ��������Ƕ�����������Ag���ɣ�����Ԫ�ػ��ϼ۱仯֪�����л��ϼ����ߵ��������ɣ�ֻ�������������Ը÷�Ӧ����ʽΪ2AgNO3$\frac{\underline{\;\;��\;\;}}{\;}$2Ag+2NO2��+O2�������������ͨ��NaOH��Һʱ��������Ӧ4NO2+O2+2H2O=4HNO3�����ݸ÷�Ӧ����ʽ֪��������ʣ�࣬����C���ռ���������������
��Ϊװ���к��п�����Ӧ�ý�װ���п����ų�ʱ���ռ����壬����Ҫ�ռ������ĸ����壬Ӧ�õȵ������������ݾ��ȷų�ʱ���ռ���
�ʴ�Ϊ���������ȵ������������ݾ��ȷų�ʱ���ռ���
��3�����ݣ�2���ķ�����д����ʽ2AgNO3$\frac{\underline{\;\;��\;\;}}{\;}$2Ag+2NO2��+O2����
�ʴ�Ϊ��2AgNO3$\frac{\underline{\;\;��\;\;}}{\;}$2Ag+2NO2��+O2����
��4���������ֽ�����в��������������ܺ�NO��Ӧ�ģ����Բ����ų�������
�ʴ�Ϊ�����������ֽ�����������
II���������Fe������ΪFe2+��
��1���ڼ���Fe3+��Fe2+ǰӦ�ó�ȥAg+�ĸ��ţ����Ե�һ��Ϊ��ȡ������Һ���Թ��У�����Һ�м�������NaCl��Һ��������ӦNaCl+AgNO3=AgCl��+NaNO3���������������г������ɣ�
��2��Fe3+��KSCN��Һ����������ʹ��Һ��Ѫ��ɫ���ڶ�����ȡ����������Һ���Թ��м���KSCN��Һ����Һ���Ѫ��ɫ��˵����Һ�к���Fe3+��
��3��Fe2+���л�ԭ�ԣ��ܱ�ǿ���������Ը��������Һ������ʹ���Ը��������Һ��ɫ����������ȡ��1��������������Һ���Թ��У���������KMnO4��Һ����Һ��ɫ��˵������Fe2+��
�ʴ�Ϊ��Fe2+��
| ���� | ���� | ���ۻ�Ŀ�� |
| ��1��ȡ������Һ���Թ��У�����Һ�м�������NaCl��Һ | - | ���� Ag+ |
| ��2��ȡ����������Һ���Թ��м��� KSCN��Һ���� | ��Һ��Ѫ��ɫ | ���� Fe3+ |
| ��3��ȡ��1��������������Һ���Թ��У���������KMnO4��Һ���� | ��ɫ��ȥ | ���� Fe2+ |
���� ���⿼������ʵ�鷽����ƣ�Ϊ��Ƶ���㣬���ؿ������ʼ��顢ʵ�������֪ʶ�㣬��ȷʵ��ԭ���ǽⱾ��ؼ����ѵ���II��ʵ�����������ƣ�Ҫ�������ʵ�����ѡȡ���ʵ�ҩƷ��ע�⻯ѧ�������ȷ���ã�Ϊ�״��㣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��ԭ��ʧȥһ�����Ӻ����ĵ���������ԭ����ͬ�����Ա����ԭ�� | |
| B�� | �κ�Ԫ�ص�ԭ�Ӷ����ɺ�����Ӻͺ������ӡ�������ɵ� | |
| C�� | ����ָһ��ԭ�ӣ���Ԫ����ָһ��ԭ�ӣ����ؼ以��ͬλ�أ�Ԫ�ذ���ͬλ�� | |
| D�� | ${\;}_{18}^{40}$Ar��${\;}_{19}^{40}$K��${\;}_{20}^{40}$Ca����������ͬ�����������ǻ�Ϊͬλ�� |
| ���� | ���� |
 | 1��A����Һ��� 2���Ժ�A����Һ�ɺ�ɫ��Ϊ��ɫ |
��2��B�з�Ӧ�����ӷ���ʽ��Cl2+2OH-�TCl-+ClO-+H2O��
��3��Ϊ̽������2������ԭ��С���������ʵ�飺
��ȡA�л�ɫ��Һ�������Թ��У�����NaOH��Һ���к��ɫ�������ɣ���ԭ��Һ��һ������Fe3+��
��ȡA�л�ɫ��Һ�������Թ��У����������KSCN��Һ�����յõ���ɫ��Һ����С��ͬѧ�˵ó����ۣ���������2��ԭ����SCN-��Cl2�����˷�Ӧ��
��4����С��ͬѧ����SCN-���ܱ�Cl2�����ˣ������ֽ���������̽����
��ȡA�л�ɫ��Һ���Թ��У������������ữ��BaCl2��Һ��������ɫ�������ɴ�֤��SCN-�б�������Ԫ����SԪ�أ�
����ʵ����SCN-�е�Ԫ�ر�����ΪNO3-������NO3-���ڵķ�����ȡ����ͭ�����Թ��У�����A�л�ɫ��Һ��һ������ϡ���ᣬ���ȣ��۲쵽�Թ��Ϸ��к���ɫ�������ɣ���֤��SCN-�е�Ԫ�ر�����ΪNO3-��
����֪SCN-�и�ԭ�Ӿ�����8�����ȶ��ṹ����д��SCN-�ĵ���ʽ
 ��
����5����SCN-��Cl2��Ӧ����1molCO2����ת�Ƶĵ�������16mol��
| A�� | Ԫ�ص���Ҫ���ϼ۶���+2�� | |
| B�� | Ԫ�صĵ��ʶ�������ɫ | |
| C�� | �е�Ԫ�صĵ��ʿ�����Ȼ�����ȶ����� | |
| D�� | ���а�������Ϊ��������������Ԫ�� |
| A�� | ʯ�͵IJ��� | B�� | ��ϩ�IJ��� | C�� | ����IJ��� | D�� | �ϳ���ά�IJ��� |
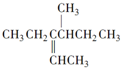 ��������ȷ���ǣ�������
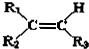
��������ȷ���ǣ�������| A�� | 3-�춡��-2-���� | B�� | 4-��-3-�һ�-2-��ϩ | ||
| C�� | 3-��-4-��ϩ������ | D�� | 2��3-���һ�-3-��ϩ |
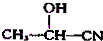 $��_{H_{2}O}^{HCl}$
$��_{H_{2}O}^{HCl}$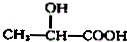
 $��_{��Zn/H_{2}O}^{��O}$
$��_{��Zn/H_{2}O}^{��O}$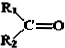 +
+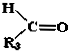
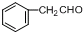 ����������ת����ϵ��
����������ת����ϵ��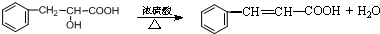 ����Ӧ����Ϊ��ȥ��Ӧ��
����Ӧ����Ϊ��ȥ��Ӧ��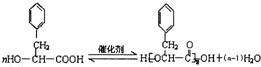 ��
��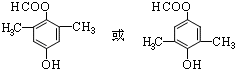 ��
��