��Ŀ����
17�������£��ס��ҡ�����λͬѧ��ʵ����ȷ��ij��HA��������ʵķ����ֱ��ǣ��ף���pH��ֽ�ⶨ0.1mol/LHA��Һ��pH������֤��HA��������ʣ�
�ң��ٷֱ�ȡpH=1��HA��Һ��ϡ�����10.00mL���ڼ���ˮϡ��Ϊ100mL��
�ڸ�ȡ��ͬ���������ϡ��Һ����������ͬʱ�ֱ���봿�Ⱥ���״��С����ͬ��п�������������۲�������֤��HA��������ʣ�
������������HA��Һ��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±��������������ݿ���˵��HA��������ʣ�
| ��� | NaOH/mol•L-1 | HA/mol•L-1 | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | C | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH��7 |
��2�����ҵķ����ĵڢٲ��У���Ҫ�õ��Ķ�����������ʽ�ζ��ܣ�pH��Ϊ1��HA��Һ��ϡ�����У�ˮ�Ķ����̶ȵĴ�С��ϵ��c������ĸ����
a��HA��Һ��ˮ�Ķ����̶ȴ�b��ϡ������ˮ�Ķ����̶ȴ�c��������Һ��ˮ�Ķ����̶���ͬ
��3���ҵķ����У�˵��HA��������ʵ���Ҫ������B������ĸ����
A��װϡ������Թ��зų�H2���ٶȿ�
B��װHA��Һ���Թ��зų�H2�����ʿ�
C�������Թ��д�ʱ�����������һ����
��4�����ķ����У���Ţ��е�c�������������������=����0.1���û��Һ�е�����Ũ�ȣ�c��Na+��=�����������������=����c��A��-��
��5�����ķ����У���Ţ۵����ݱ����������Һ��HA�ĵ���̶ȱ�NaA��ˮ��̶ȣ�ǿ���ǿ��������������ȷ��������
���� ��1��������ʵĵ����ǿ���ģ�������ȫ���룻
��2����ȷȡpH=1��HA��Һ��ϡ�����10.00mL������ʽ�ζ��ܣ�pH��Ϊ1��HA��Һ��ϡ�����У�˵��������Ũ����ȣ�����ˮ�����ӻ����õ�����������Ũ����ȣ�����������Һ��ˮ�Ķ����̶���ͬ��
��3��������ˮ��Һ�ﲿ�ֵ��룬����������Ũ��С����Ũ�ȣ����п��Ӧʱ������������Ӧ���ʺ�������Ũ�ȳ����ȣ��������������ķ�Ӧ����ȷ�����ǿ����
��4������Ϊǿ�ᣬ�������Ũ�Ȼ��ʱpH=7����HAΪ���ᣬ�������Ũ�Ȼ����Һ��pH����7����Ϊ��֤pH=7��Ӧʹ��Ũ�ȴ���0.1mol/L������ϵ���غ��������Ũ�ȹ�ϵ��
��5���ɢ���ʵ������֪����Ϻ�ΪHA��NaA�Ļ��Һ��pH=7����ĵ�������ε�ˮ�⣮
��� �⣺��1������0.1mol/L��HA����Һ100mL��������ʵĵ����ǿ���ģ�������ȫ���룬��pH��ֽ�������Һ��pHֵ�������pH��1��Ϊ���ᣬpH=1��Ϊǿ�ᣬ�Ȱ�һС��pH��ֽ���ڱ��������Ƭ�ϣ����ò�����պȡ��Һ������ֽ���в�������ɫ�������ɫ���Ա�ȷ����Һ��pH��
�ʴ�Ϊ�������Ȱ�һС��pH��ֽ���ڱ��������Ƭ�ϣ����ò�����պȡ��Һ������ֽ���в�������ɫ�������ɫ���Ա�ȷ����Һ��pH��
��2����ȷȡpH=1��HA��Һ��ϡ�����10.00mL������ʽ�ζ��ܣ�pH��Ϊ1��HA��Һ��ϡ�����У�˵��������Ũ����ȣ�����ˮ�����ӻ����õ�����������Ũ����ȣ�����������Һ��ˮ�Ķ����̶���ͬ���ʴ�Ϊ����ʽ�ζ��ܣ�c��
��3��pH��ȵ�һԪ�ᣬ�����Ũ�ȴ���ǿ�ᣬ�������PH�Ĵ��������ϡ����ͬ����ʱ��������������Ũ�ȴ������ᣬ�ֱ��п��Ӧʱ���������������ķ�Ӧ���ʴ���ǿ�ᣬ��B��ȷ��
�ʴ�Ϊ��B��
��4������Ϊǿ�ᣬ�������Ũ�Ȼ��ʱpH=7����HAΪ���ᣬ�������Ũ�Ȼ����Һ��pH����7����Ϊ��֤pH=7��Ӧʹ��Ũ�ȴ���0.1mol/L��
�ɵ���غ��֪c��Na+��+c��H+��=c��A-��+c��OH-����c��H+��=c��OH-������c��A-��=c��Na+����
�ʴ�Ϊ������=��
��5���ɢ���ʵ������֪���������Ϻõ�ͬŨ��HA��NaA�Ļ��Һ��pH��7��˵����ĵ���̶ȴ����ε�ˮ��̶ȣ��ʴ�Ϊ��ǿ��
���� �⿼���������ҺpH�ļ��㼰����Ũ�ȴ�С�ıȽϣ���ȷ����Ϻ���Һ�е����ʡ�����ˮ�⡢�����Ũ����pH�Ĺ�ϵ���ɽ����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | Ǧ���������͵���ɫ������� | |
| B�� | ����·��ת��0.2mol����ʱ�����ĵ�H2SO4Ϊ0.2 mol | |
| C�� | Ǧ���طŵ�ʱ����������С�������������� | |
| D�� | Ǧ���طŵ�ʱ�����ɸ���������Һ�����ƶ������� |
��������
| A�� | 2H2��g��+O2��g���T2H2O ��l����H=-285.8 kJ•mol-1 | |
| B�� | 2H2��g��+O2��g���T2H2O ��l����H=+571.6 kJ•mol-1 | |
| C�� | 2H2��g��+O2��g���T2H2O ��g����H=-571.6 kJ•mol-1 | |
| D�� | H2��g��+1/2O2��g���TH2O ��l����H=-285.8 kJ•mol-1 |
| A�� | x��m��n=1��2��1 | B�� | m��n=2��1 | C�� | m��2x+2 | D�� | m=2 |
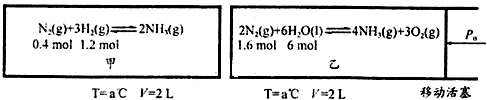

 ��
��