��Ŀ����
12������������һ��������Ư�������㷺Ӧ����ϴ�·ۣ�Ư�ۺ�ϴ�Ӽ��У�����ɰ����Ҫ�ɷ�Na2B4O7•10H2O��Ϊԭ�������������ƾ������Ҫ�������£�
��1����Na2B4O7��NaOH��Ӧ��NaBO2�����ӷ���ʽΪB4O72-+2OH-=4BO2-+H2O��
��2������A ��������Mg��OH��2�������������ɵú�Mg2+����Һ�����˺�����Һ�мӼ��γ�Mg��OH��2����c��Mg2+��=0.056mol•L-1����ô����pH=9ʱ���ſ�ʼ����Mg��OH��2����������֪��Mg��OH��2��Ksp=5.6��10-12��
��3���������������ֺ�ˮ�Σ���A��NaBO3•4H2O��B��NaBO3•H2O��д����A�Ƶ�B�ķ���ʽ��ָ��������NaBO3•4H2O$\frac{\underline{\;����70��\;}}{\;}$NaBO3•H2O+3H2O��A��B��һ����Ư������ǿB��
��4���ù�̼���ƣ�2Na2CO3•3H2O2�������������Ư����ʲô��ȱ���̼���ƽϹ��������ڵ������ܽ����ܺã��۸�ߣ�
���� ����ɰ����Ҫ�ɷ�Na2B4O7•10H2O��Ϊԭ�������������ƾ��壬��ɰ�м�������������Һ������ҺPH=10-11�����˵õ�����A��NaBO2����Һ���������������˵õ������ƾ��壬
��1��������֪��Na2B4O7��NaOH��Ӧ��NaBO2�����ԭ���غ�͵���غ㼴��д�����ӷ���ʽ��
��2�������ܶȻ����������������Ũ�ȣ��ٸ���ˮ�����ӻ���������������Ũ�ȣ�����pH��
��3�����������Ŀ����У����ȽϸߵĹ��������ǽ��ȶ��ģ���40���ʪ�Ŀ����зֽ⣬���ų���������63��ʱ���������Ľᾧˮ���ֽ⣬�������Թ̿飬����70��ʱʧȥ3���ᾧˮ���γ�һˮ�һˮ����������Ϊһ�ָ�Ч�ȶ�����ϵƯ����
��4������������60�����ϵ���ˮ�в��ܳ���ܽⲢ�������ã���̼���ƽϹ��������ڵ������ܽ����ܺã�
��� �⣺��1��������֪��д����Ӧ��Ͳ��������
Na2B4O7+NaOH��NaBO2+�������������غ��֪δ֪��ΪH2O��
��ƽ��ʽΪ��Na2B4O7+2NaOH�T4NaBO2+H2O�����ӷ���ʽΪ��B4O72-+2OH-=4BO2-+H2O��
�ʴ�Ϊ��B4O72-+2OH-=4BO2-+H2O��
��2��Mg2+������PH�������£�Ksp�Tc��Mg2+����c��OH-��2 ����c��OH-��Ũ��Ϊx������Ksp����ʽ�ã�
0.056��x2�T5.6��10-12��
���x�T1.0��10-5��
C��H+��=$\frac{1��1{0}^{-14}}{1��1{0}^{-5}}$�T1��10-9��
pH�T9���ʵ�����Һ��pH=9ʱ���ſ�ʼ���ֳ�����
�ʴ�Ϊ��9��
��3�����������Ŀ����У����ȽϸߵĹ��������ǽ��ȶ��ģ���40���ʪ�Ŀ����зֽ⣬���ų���������63��ʱ���������Ľᾧˮ���ֽ⣬�������Թ̿飬����70��ʱʧȥ3���ᾧˮ���γ�һˮ���Ӧ�Ļ�ѧ����ʽΪ��NaBO3•4H2O$\frac{\underline{\;����70��\;}}{\;}$NaBO3•H2O+3H2O��һˮ�������Ǵ���ˮ����Ĺ����������ѵ�����ˮ���Ӻ�õ���Ʒ������ˮ����������ȣ�һˮ�������ƾ��и��ߵĻ��������������ߵ����ȶ��Ժ��ߵ��ܽ����ʣ�
�ʴ�Ϊ��NaBO3•4H2O$\frac{\underline{\;����70��\;}}{\;}$NaBO3•H2O+3H2O��B��
��4������������60�����ϵ���ˮ�в��ܳ���ܽⲢ�������ã���̼���ƽϹ��������ڵ������ܽ����ܺã���ˮ��Һ�ʼ��ԣ����Էֽ�Ϊ̼���ƺ������⣬�и��õ�Ư��Ч�����ʴ�Ϊ����̼���ƽϹ��������ڵ������ܽ����ܺã��۸�ߣ�
���� ���⿼��������ѡ������жϣ��������ѵ㣬����ʱҪ���������Ŀ����������ϵ�ͱ������ݣ�����������ϵ�����������ĸ�����ٽ��⣮

| A�� | ��ԭ�ӵĽṹʾ��ͼ�� | |
| B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | |
| C�� | ���������ӵĵ���ʽ�� | |
| D�� | ������Ϊ146��������Ϊ92 ���ˣ�U��ԭ�ӣ�${\;}_{92}^{146}$U |

| A�� | ʯīת��Ϊ���ʯ�����ȷ�Ӧ | |
| B�� | CO��g��+H2O��g���TCO2��g��+H2��g�������ȷ�Ӧ | |
| C�� | ��ͬ�����£�������S��g����S��s���������Ƚϣ�S��s���ϴ� | |
| D�� | ���ױȺ����ȶ� |
| A�� | Mg2+��OH-��NH4+��Na+ | |
| B�� | c��H+��ˮ=1.0��10-12mol•L-1����Һ�У�S2-��Na+��OH-��K+ | |
| C�� | S2-��Cl-��Al3+��SO42- | |
| D�� | S2-��K+��Fe3+��Cl- |
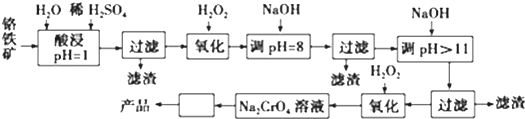
��֪����+3��Cr��������Һ�������ȶ�����pH��9ʱ��CrO2-��ʽ��������������
�ڳ����£�����������������������ʽ����ʱ��Һ��pH���£�
| ������ | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cr3+ |
| ��ʼ����ʱ��pH | 2.7 | 7.6 | 9.0 | -- | -- |
| ������ȫʱ��pH | 3.7 | 9.6 | 11.0 | 8 | 9����9�ܽ⣩ |
��2������ͼ�С������ڵIJ���������Ũ������ȴ�ᾧ��
��3������NaOH������Һ��pH=8ʱ������ȥ��������Fe3+��Al3+��������Һ��pH��11ʱ������ȥ��������Mg2+��
��4������pH=8���͡���pH��11���м�ġ����ˡ������ܷ�ʡ�ԣ�Ϊʲô�����ܣ���pH=8ʱAl3+�Ѿ���ȫת��ΪAl��OH��3�����������˳�ȥ������������NaOHʱAl��OH��3���ܽ⣬������������AlO2-��
�ף���pH��ֽ�ⶨ0.1mol/LHA��Һ��pH������֤��HA��������ʣ�
�ң��ٷֱ�ȡpH=1��HA��Һ��ϡ�����10.00mL���ڼ���ˮϡ��Ϊ100mL��
�ڸ�ȡ��ͬ���������ϡ��Һ����������ͬʱ�ֱ���봿�Ⱥ���״��С����ͬ��п�������������۲�������֤��HA��������ʣ�
������������HA��Һ��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±��������������ݿ���˵��HA��������ʣ�
| ��� | NaOH/mol•L-1 | HA/mol•L-1 | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | C | 0.2 | pH=7 |
| �� | 0.1 | 0.2 | pH��7 |
��2�����ҵķ����ĵڢٲ��У���Ҫ�õ��Ķ�����������ʽ�ζ��ܣ�pH��Ϊ1��HA��Һ��ϡ�����У�ˮ�Ķ����̶ȵĴ�С��ϵ��c������ĸ����
a��HA��Һ��ˮ�Ķ����̶ȴ�b��ϡ������ˮ�Ķ����̶ȴ�c��������Һ��ˮ�Ķ����̶���ͬ
��3���ҵķ����У�˵��HA��������ʵ���Ҫ������B������ĸ����
A��װϡ������Թ��зų�H2���ٶȿ�
B��װHA��Һ���Թ��зų�H2�����ʿ�
C�������Թ��д�ʱ�����������һ����
��4�����ķ����У���Ţ��е�c�������������������=����0.1���û��Һ�е�����Ũ�ȣ�c��Na+��=�����������������=����c��A��-��
��5�����ķ����У���Ţ۵����ݱ����������Һ��HA�ĵ���̶ȱ�NaA��ˮ��̶ȣ�ǿ���ǿ��������������ȷ��������
| A�� | 1molFeI2������������Ӧʱת�Ƶĵ�����Ϊ3NA | |
| B�� | 1L2mol•L-1 K2S��Һ��S2-��HS-������Ϊ2NA | |
| C�� | ��״���£�22.4L��CCl4�к��е�CCl4������ΪNA | |
| D�� | 50mL18mol•L-1Ũ����������ͭ�ȷ�Ӧ��ת�Ƶĵ�����Ϊ1.8NA |
�ٸʰ��� �ڱ����� �۱������� �ܹȰ���

| A�� | �٢� | B�� | �ۢ� | C�� | �ڢ� | D�� | �٢� |
| A�� | ������̼��ʯ��ˮ��Ӧ��CO2+2OH-�TCO32-+H2O | |
| B�� | ��ˮ��ͨ��������SO2��Br2+SO2+2H2O�T2Br-+SO42-+4H+ | |
| C�� | Cu����ϡ���Cu++2H++NO3-�TCu2++NO2��+H2O | |
| D�� | Na2O2����ˮ����O2��Na2O2+H2O�T2Na++2OH-+O2�� |