��Ŀ����
����Ŀ�������Ȼ�ѧ����ʽ��ȷ����
A. ����ı�ȼ����Ϊ890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����890.3 kJ��mol��1
B. 500 �桢30 MPa �£���0.5 mol N2 �� 1.5 mol H2 �����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪ��N2(��)+3H2(g) ![]() 2NH3(g)��H����38.6 kJ��mol��1
2NH3(g)��H����38.6 kJ��mol��1
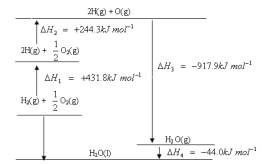
C. ��֪��120 �桢101 kPa�£�1 g H2ȼ������ˮ�����ų�121 kJ���������Ȼ�ѧ����ʽΪ��H2(g)��![]() O2(g)
O2(g)![]() H2O(g)����H����242 kJ/mol
H2O(g)����H����242 kJ/mol
D. CO(g)��ȼ������283.0 kJ��mol��1����2CO2(g)===2CO(g)��O2(g)��Ӧ�Ħ�H��+283.0 kJ��mol��1
���𰸡�C
��������
A.ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų��������������ȼ����Ϊ890.3kJmol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4��g��+2O2��g���T2CO2��g��+2H2O��l����H=-890.3kJmol-1��ˮ��������̬����A����
B.���ĺϳ�Ϊ���淴Ӧ��0.5 molN2��1.5molH2�����ܱ������г�ַ�Ӧ����NH3(g)������19.3kJ����ӦN2(��)+3H2(g) ![]() 2NH3(g)�ķ�Ӧ����H����38.6kJ��mol��1����B����
2NH3(g)�ķ�Ӧ����H����38.6kJ��mol��1����B����
C.1gH2ȼ������ˮ�����ų�121kJ��������1molH2ȼ�շ���242kJ�������Ȼ�ѧ����ʽΪ��H2(g)��1/2O2(g)![]() H2O(g) ��H����242kJ/mol����C��ȷ��
H2O(g) ��H����242kJ/mol����C��ȷ��
D.CO(g)��ȼ������283.0kJ��mol��1����CO(g)��1/2O2(g)=CO2(g) ��H����283.0 kJ��mol��1����2CO2(g)=2CO(g)��O2(g)��Ӧ����H��+2��283.0kJ��mol��1������Ӧʱ��Ӧ�ȵ���ֵ��ȣ������෴����D����
��ѡC��