��Ŀ����
20��KI����������Ⱦ�ϡ��й���ϡ�ʳƷ���Ӽ��ȣ��乤ҵ�����������£�
��1�����绯������֮һ�ǵ���أ�KIO3�����÷�Ӧ�����ӷ���ʽ��I2+6OH-�TI-+IO3-+3H2O��
��2������ԭ��������ʹ�õ���м���ü���Һ��ϴ����Ŀ����ȥ����м��������ۣ�
��3������pH���ľ���ʵ���������ԭ�����û�����еμ�KOH��Һ���ýྻ�IJ�����պȡ������Һ������pH��ֽ�ϣ��������ɫ�����գ����pH���ظ�������ֱ��pH��9�������Լ���KOH��Һ��pH��ֽ����
��4��������X���������ǹ��ˣ�Y�Ļ�ѧʽ��Fe��OH��2��Fe��OH��3��
��5���ڡ�����X����Ϊ�õ�KI���壬����Ҫ���е�ʵ�����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ�
��6���ڲⶨ��Ʒ��KI����ʱ������京��Ϊ101.5%����ԭ������Dz�Ʒ�л���I2�������Ʒ�к�I2�ķ�����ȡ������Ʒ���Թ��У�������ˮ�ܽ⣻�ٵ��������Һ������Һ����ɫ��˵����Ʒ�л���I2��
���� �ⵥ���ڼ��������·����绯��Ӧ���ɵ⻯�غ͵���أ�������ϵ�м������ۻ�ԭ��������ӣ�����������Ϊ�����ӣ�Ȼ����������ص�����Һ��pH��ʹ�����Ӻ������ӳ�����������Һ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ����յõ��⻯�ƣ�
��1���ⵥ���ڼ�����Һ�з���������������ԭ��Ӧ�����ɵ����Ӻ͵�������ӣ�
��2����м���������ۣ��ڼ��������³�ȥ���ۣ�ʹ�������س�ֽӴ�������������ԭ��Ӧ��
��3���ⶨ��Һ��pH���ýྻ�IJ�����պȡ������Һ������pH��ֽ�ϣ��������ɫ�����գ����pH��ֱ�������pHԼΪ9��
��4�����������Һ��Ļ�����ù��ˣ�������۹������������������������������������������������������ߵĻ���
��5�����⻯����Һ�����ʷ�������ķ�������⻯�Ƶ��ܽ�����¶ȵ�Ӱ��仯�ܴ�����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵý�Ϊ�����ĵ⻯�ƣ�
��6������ⵥ�ʵĴ��ڼӵ��ۣ���Ϊ�ⵥ����ʹ���۱�����
��� �⣺��1���ⵥ���ڼ�����Һ�з���������������ԭ��Ӧ�����ɵ����Ӻ͵�������ӣ����ӷ���ʽΪ��I2+6OH-�TI-+IO3-+3H2O���ʴ�Ϊ��I2+6OH-�TI-+IO3-+3H2O��
��2����м���������ۣ��ڼ��������³�ȥ���ۣ�ʹ�������س�ֽӴ�������������ԭ��Ӧ���ʴ�Ϊ��ȥ����м��������ۣ�
��3���ⶨ��Һ��pH���ýྻ�IJ�����պȡ������Һ������pH��ֽ�ϣ��������ɫ�����գ����pH��ֱ�������pHԼΪ9���ʴ�Ϊ����ԭ�����û�����еμ�KOH��Һ���ýྻ�IJ�����պȡ������Һ������pH��ֽ�ϣ��������ɫ�����գ����pH���ظ�������ֱ��pH��9��
��4�����������Һ��Ļ�����ù��ˣ�������۹�������������Ϊ�������Ӽ�����������������������������������Ϊ���������ӣ��Ӽ�����������������������������ߵĻ����ʴ�Ϊ�����ˣ�Fe��OH��2��Fe��OH��3��
��5�����⻯����Һ�����ʷ�������ķ�������⻯�Ƶ��ܽ�����¶ȵ�Ӱ��仯�ܴ�����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵý�Ϊ�����ĵ⻯�ƣ��ʴ�Ϊ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ�
��6������ⵥ�ʵĴ��ڼӵ��ۣ���Ϊ�ⵥ����ʹ���۱���������IJ���Ϊ��ȡ������Ʒ���Թ��У�������ˮ�ܽ⣻�ٵ��������Һ������Һ����ɫ��˵����Ʒ�л���I2���ʴ�Ϊ��ȡ������Ʒ���Թ��У�������ˮ�ܽ⣻�ٵ��������Һ������Һ����ɫ��˵����Ʒ�л���I2��
���� ���⿼�����ɵ��Ƶõ⻯�ƵĹ������̼����ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ��漰֪ʶ��϶࣬��ȷ������������Ϊ�����Ĺؼ�������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

| A�� | ��ϩ�Ӿ� | B�� | ��ϩ��ˮ�ӳ� | C�� | ��ϩ��Br2�ӳ� | D�� | ������Br2��ȡ�� |
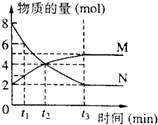
| A�� | ��Ӧ����ʽΪN?2M | |
| B�� | t2ʱ�����淴Ӧ������� | |
| C�� | �����������䣬��С�������������Ӧ���ʻ�ӿ� | |
| D�� | ��t1=1����0��t1ʱ���N��ƽ������Ϊ3mol/��L•min�� |
| A�� | ȡ��-��ȥ-�ӳ�-���� | B�� | ��ȥ-�ӳ�-ȡ��-���� | ||
| C�� | ��ȥ-ȡ��-�ӳ�-�� | D�� | ȡ��-�ӳ�-����-��ȥ |
C2H5OH��g���TC2H5OH��l����H2=-b kJ/mol
H2O��g���TH2O��l����H3=-c kJ/mol
��ʹ92g�ƾ�Һ����ȫȼ�գ����ָ������£���ų�����������λkJ��Ϊ��������
| A�� | 4a+4b+4c | B�� | 2a-2b+6c | C�� | 2a-2b+2c | D�� | 2a-6b+2c |
| A�� | �����£�0.1 mol•L-1��������Һ�к���Na+��CH3COO-������Ϊ0.2NA | |
| B�� | �����£���0.1 mol��Ƭ��������Ũ�����з�Ӧ��ת�Ƶ��ӵ���ĿΪ0.3NA | |
| C�� | 2 g NO2��44 g N2O4�Ļ������������ԭ�ӵ�����Ϊ3NA | |
| D�� | 0.1 mol����ȩ�к���˫������ĿΪ0.3NA |
| A�� | �٢ڢۢܢ� | B�� | �ڢۢܢݢ� | C�� | �ܢݢڢ٢� | D�� | �ۢ٢ڢݢ� |
 ��һ�ܱ������м���A��B�������ʵ����ʵ���Ũ�����ŷ�Ӧ�Ľ��У���ͼ��ʾ������˵������ȷ���ǣ�������
��һ�ܱ������м���A��B�������ʵ����ʵ���Ũ�����ŷ�Ӧ�Ľ��У���ͼ��ʾ������˵������ȷ���ǣ�������| A�� | �÷�Ӧ�Ļ�ѧ����ʽΪ5A+4B?4C | |
| B�� | 2minʱ���÷�Ӧ�ﵽƽ�⣬��ʱA��B��C��Ũ�ȱ�Ϊ5��4��4 | |
| C�� | ��B��Ũ�ȱ仯��ʾ2min�ڵ�����Ϊ2mol/��L•min�� | |
| D�� | 2minǰ������Ӧ������С���淴Ӧ���������� |