��Ŀ����
20��Ŀǰ�Ѿ���Ϊ��϶���������һ����Ҫ������ͣ�NiMH�е�M��ʾ���������Ͻ𣮸õ���ڳ������е��ܷ�Ӧ����ʽ�ǣ�Ni��OH��2+M=NiOOH+MH����֪��6NiOOH+NH3+H2O+OH-=6Ni��OH��2+NO2-������˵����ȷ���ǣ�������| A�� | NiMH��طŵ�����У������ĵ缫��ӦʽΪNiOOH+H2O+e-=Ni��OH��2+OH- | |
| B�� | ��������OH-���Ӵ�����������Ǩ�� | |
| C�� | �������������ĵ缫��Ӧʽ��H2O+M+e-=MH+OH-��H2O�е�H��M��ԭ | |
| D�� | NiMH����п�����KOH��Һ����ˮ����Ϊ�������Һ |
���� ����������ҪΪKOH�����Һ���ʱ��������Ӧ��Ni��OH��2+OH--e-=NiOOH+H2O��������Ӧ��M+H2O+e-=MH+OH-���ܷ�Ӧ��M+Ni��OH��2=MH+NiOOH��
�ŵ�ʱ��������NiOOH+H2O+e-=Ni��OH��2+OH-��������MH+OH--e-=M+H2O���ܷ�Ӧ��MH+NiOOH=M+Ni��OH��2������ʽ��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ�
A���������Ϸ�����д�����缫��Ӧʽ��
B���������У������������������ƶ���
C�����ʱ�������ϵõ��ӷ�����ԭ��Ӧ��
D���а�������ʱ��������Ӧ��
��� �⣺����������ҪΪKOH�����Һ ���ʱ��������Ӧ��Ni��OH��2+OH-=NiOOH+H2O+e-��������Ӧ��M+H2O+e-=MH+OH-���ܷ�Ӧ��M+Ni��OH��2=MH+NiOOH��
�ŵ�ʱ��������NiOOH+H2O+e-=Ni��OH��2+OH-��������MH+OH-=M+H2O+e-���ܷ�Ӧ��MH+NiOOH=M+Ni��OH��2������ʽ��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ�
A�������ĵ缫��ӦʽΪ��NiOOH+H2O+e-�TNi��OH��2+OH-����A��ȷ��
B�����ʱ�������������ƶ����������������ƶ�������OH-���Ӵ���������������B����
C��H2O�е�H�õ��ӣ����DZ�M��ԭ����C����
D�������ð�ˮ���������Һ����ΪNiOOH�ܺͰ�ˮ������Ӧ����D����
��ѡA��
���� ���⿼����ԭ��غ͵���ԭ������ȷ�����Ϣ�ĺ����ǽⱾ��ؼ����ѵ�ĵ缫��Ӧʽ����д����Ŀ�Ѷ��еȣ�

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�| A�� | ����Һ�����ԣ���c��K+��=c��CH3COO-�� | |
| B�� | ����Һ�ʼ��ԣ���c��K+����c��CH3COO-�� | |
| C�� | ����Һ�����ԣ���c��H+����c��CH3COO-����c��K+�� | |
| D�� | ��Һ�ʵ����ԣ���c��H+��+c��K+��=c��CH3COO-��+c��OH-�� |
| A�� | CH4���ӵĽṹʽ��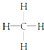 | B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | ||
| C�� | F-�ṹʾ��ͼ��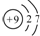 | D�� | �����ӵĵ���ʽ��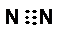 |
| A�� | ���ȩ������ԭ����Ư�ۡ���������������ͬ | |
| B�� | 1 mol ���ȩ���ӿɱ�1 mol Cu��OH��2��ȫ���� | |
| C�� | CH3CH�TCHCH2COOH�����ȩ��Ϊͬ���칹�� | |
| D�� | 10g���ȩ��ȫȼ��������0.6 mol O2 |
| A�� | Fe2O3 | B�� | MnO2 | C�� | CaO | D�� | V2O5 |
��CH3OH��g��+H2O��g���TCO2��g��+3H2��g������H=+49.0kJ/mol
��CH3OH��g��+$\frac{1}{2}$O2��g���TCO2��g��+2H2��g������H=-192.9kJ/mol
����˵����ȷ���ǣ�������
| A�� | CH3OH��ȼ��Ϊ���ȷ�Ӧ | |
| B�� | 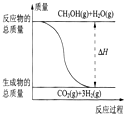 ��Ӧ���е������仯��ͼ��ʾ | |
| C�� | CH3OHת���H2�Ĺ���һ��Ҫ�������� | |
| D�� | ���ݢ���֪��Ӧ��CH3OH��l��+$\frac{1}{2}$O2��g���TCO2��g��+2H2��g���ġ�H��-192.9kJ/mol |
| A�� | ú�ĸ����ʯ�͵ķ��������ѧ�仯 | |
| B�� | �����ʺ����Ƕ����ڸ߷��ӻ����һ�������¶���ˮ�� | |
| C�� | ��ϩˮ�����Ҵ���ԭ��������Ϊ�ٷ�֮�٣�������ɫ��ѧԭ�� | |
| D�� | ��������ά�ػ�Ϊͬ���칹�� |
| A�� | 100mL 2mol/L NH4NO3��Һ | B�� | 40mL 0.5mol/L Ca��NO3��2��Һ | ||
| C�� | 50mL 1.5mol/L Al��NO3��3��Һ | D�� | 150mL 1mol/L Mg��NO3��2��Һ |
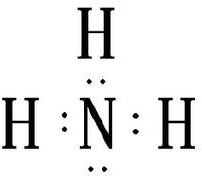 ��
��