��Ŀ����
����Ŀ���ش��������⣺
��1�������£���m mol��L��1��CH3COOH��Һ��n mol��L��1NaOH��Һ�������Ϻ���Һ��pH��7����m��n�Ĵ�С��ϵ��m____n�����������������=������ͬ����ԭ����c(H+)�����c(OH-)�Ĵ�С��ϵ��c(H+)____c(OH-)��
��2�������£���Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COONa��CH3COOH��Һ�������Ϻ���Һ�����ԡ�������Һ�и�����Ũ���ɴ�С����Ϊ_____�����ڸû����Һ��˵������ȷ����___(�����)��
a������Һһ����c(Na+)+c(H+)=c(OH��)+c(CH3COO��)
b������Һһ����c(Na+)=c(CH3COOH)+c(CH3COO��)
c������Һ��ˮ�ĵ���̶�һ�����ڴ�ˮ�ĵ���̶�
d�������Һ�м������������ƻ����ᣬ��ҺpH�仯����
��3����0.1000 mol��L��1NaOH��Һ�ζ�20.00mLijŨ�ȵ�CH3COOH��Һ���ζ���������ͼ��ʾ�����е����ʾ��Һ��c(CH3COO-)=1.7c(CH3COOH)�������ʾ��Һ��c(CH3COO-)+c(CH3COOH)=c(Na+)����ʽ���������ĵ��볣��_____��CH3COOH�����ʵ���Ũ��Ϊ____ mol��L��1��
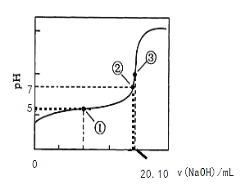
���𰸡��� �� c(CH3COO- )��c(Na+)��c(H+)��c(OH-) bc 1.7��10-5 0.1005
��������
��1��pH��7��˵��c(H+)=c(OH-)��CH3COOHΪ���ᣬ����������ǿ���˷�Ӧ������ǹ����ģ����m��n��c(H+)��c(OH-)��
��2����Ũ�Ⱦ�Ϊ0.1mol��L��1��CH3COONa��CH3COOH��Һ��������c(CH3COO-)��࣬Ũ���������c(CH3COO-)��c(Na+)����Һ�����ԣ���c(H+)��c(OH-)����ô�����Һ�и�����Ũ���ɴ�С����Ϊ��c(CH3COO-)��c(Na+)��c(H+)��c(OH-)��
a�����ݵ���غ��֪c(Na+)+c(H+)=c(OH��)+c(CH3COO��)��a����ȷ��
b�����������غ���2c(Na+)=c(CH3COOH)+c(CH3COO��)��b�����
c�������Һ�����ԣ���������Һ��ˮ�ĵ��룬�����Һ��ˮ�ĵ���̶�С�ڴ�ˮ�ĵ���̶ȣ�c�����
d���γɵĻ����ҺΪ������Һ��������������������ˮ��ٽ��˵��룬�������������˵��룬�ٽ���ˮ�⣬�����ҺpH�仯����d����ȷ��
��ѡbc��
��3�������Һ��c(CH3COO-)=1.7c(CH3COOH)����ʱpH=5��c(H+)=10-5mol/L������ĵ��볣��Ka=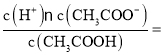 1.7��10-5��
1.7��10-5��
�����Һ��c(CH3COO-)+c(CH3COOH)=c(Na+)��˵��NaOH��Һ��CH3COOHǡ����ȫ��Ӧ�����ʱc(CH3COOH)=![]() =0.1005mol/L��
=0.1005mol/L��

 ��У����ϵ�д�
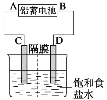
��У����ϵ�д�����Ŀ����ʽ̼����[Cox(OH)y(CO3)z]���������Ӳ��ϣ����Բ��ϵ����Ӽ�������ʱ�ɷֽ��������������Ϊ��ȷ������ɣ�ij��ѧ��ȤС��ͬѧ�������ͼ��ʾװ�ý���ʵ�顣
��1�����������ʵ�鲽�裺
�ٳ�ȡ3.65g��Ʒ����Ӳ�ʲ������ڣ������ҡ���װ�õ�������
�ڰ���ͼ��ʾװ����װ��������������װ�������ԣ�
�ۼ��ȼ��в����ܣ�����װ����________________________����ʵ������ֹͣ���ȣ�
�ܴ���a������ͨ����������Ӻ����ҡ���װ�õ��������ݼ��㡣
��2��������л���ͨ����������ӵ�Ŀ����_________________________________________��
��3��ijͬѧ��Ϊ����ʵ��װ���д���һ������ȱ�ݣ�Ϊ�����һ���⣬��ѡ������װ���е�____������ĸ��������װ��______֮ǰ��������������������������������������
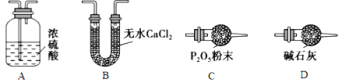
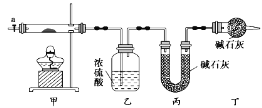
��4��������ȷװ�ý���ʵ�飬����������ݣ���ü�ʽ̼���ܵĻ�ѧʽΪ______________��
��װ�õ�����/g | ��װ�õ�����/g | |
����ǰ | 80.00 | 62.00 |
���Ⱥ� | 80.36 | 62.88 |
��5��CoCl2��6H2O���������ˮ������Ӽ����Ժ��ܷ��ϣ�������Fe��Al�����ʣ���ȡCoCl2��6H2O��һ�ֹ������£�
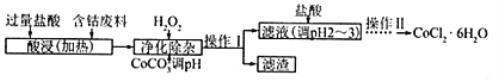
��֪��
������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 |
��ʼ����(pH) | 2.3 | 7.5 | 7.6 | 3.4 |
��ȫ������pH�� | 4.1 | 9.7 | 9.2 | 5.2 |
�پ���������ʱ������H2O2������Ӧ�����ӷ���ʽΪ__________________________________��
�ڼ���CoCO3��pH���ӵõ�����Al(OH)3��Fe(OH)3����pHӦ������_____������pHֵ�ķ�Χ��
�ۼ��������pHΪ2��3��Ŀ��Ϊ_____________________________________��
�ܲ��������Ϊ_________________________________����������ƣ������ˡ�