��Ŀ����
19��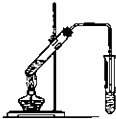 ��1��ijͬѧ����12mol/L��Ũ��������1mol/L������250mL��
��1��ijͬѧ����12mol/L��Ũ��������1mol/L������250mL���ٲ���ʱ�������õ���������BCD������ĸ��ʾ��
A.500mL����ƿ B.250mL����ƿ C.100mL�ձ� D.50mL��Ͳ E.10mL��Ͳ
�ڲ����У���δ��ϴ���ձ����ϴҺע������ƿ����������Һ�����ʵ�����Ũ��ƫС���ƫ��ƫС������Ӱ�족��
��2��ʵ��������ͼ��ʾ��װ����ȡ����������
���Ҳ��Թ��еĵ��ܲ�������Һ��Ŀ���Ƿ�ֹ������
������ߵ��Թ��м����Ƭ�������Ƿ�ֹ���У�
�����жԸ�ʵ��IJ������������AD��
A���ô��Ѹ������ B����С��С�ļ��� C����Ũ��������Ҵ��� D��������������Һ���ղ��
���� ��1���ٸ�����Һϡ��ǰ���������ʵ��������������Ũ��������������Ũ��������ѡ����ʵ���Ͳ������������Һ�����ѡ����ʵ�����ƿ��
����δ��ϴ���ձ����ϴҺע������ƿ���������ʵ����ʵ���ƫС������C=$\frac{n}{V}$������������
��2�����Ҵ������ᶼ������ˮ���ܲ���Һ������������������
�ڻ��Һ��������������У�
�������Ҵ��������ӷ���Ũ�������ȷ��ϡ�ͷ��������������ܹ�����������������ˮ����
��� �⣺��1��������12mol/L��Ũ��������1mol/L������250mL��Ӧѡ��250ml����ƿ��
����ҪŨ��������ΪV������Һϡ��ǰ���������ʵ������䣬��12mol/L��V=250mL��1mol/L
���V=20.8ml������Ӧѡ��50ml��Ͳ��
��100ml�ձ�ϡ��Ũ���
��ѡ��BCD��
����δ��ϴ���ձ����ϴҺע������ƿ���������ʵ����ʵ���ƫС������C=$\frac{n}{V}$��֪��Һ�����ʵ���Ũ��ƫС��
�ʴ�Ϊ��ƫС��
��2���ٻӷ�������������Ҵ�������̼������Һ�����Ե��ܲ��ܲ���Һ���£�ԭ���Ƿ�ֹ������
�ʴ�Ϊ����ֹ������
���Ҵ������ᡢŨ������Һ�������������У��������Ƭ���Է�ֹ���з�����
�ʴ�Ϊ����ֹ���У�
���Ҵ���������Ũ����������£���Ӧ���������������Ҵ������ᶼ���ӷ������Կ�ʼ����ʱӦ��С��С�ļ��ȣ������Ҵ�������Ļӷ���
Ũ�������Ҵ���ϣ�Ϊ��ֹŨ����ϡ�Ͳ����������ȶ�ʹҺ�彦����Ӧ��Ũ������뵽�Ҵ��У�
���ɵ����������ڼ��Ի�����ˮ�⣬�����������������գ�Ӧ�ñ���̼������Һ���գ�
��ѡ��AD��
���� ���⿼��������һ�����ʵ���Ũ�ȡ�������������ȡ����ȷ����ԭ������Ͳ������ƿѡ��ķ�������ȷ�Ҵ������ἰ���������������ǽ���ؼ�����Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ú�ĸ����ʯ�͵ķ�������ѧ�仯 | |
| B�� | ���ᱵ��ҽѧ���������ͣ������Ӷ������� | |
| C�� | ̼-14��������������ļ�����̼-14��̼-12��Ϊͬ�������� | |
| D�� | ������ע��Һ���ܲ��������ЧӦ�������ڽ��� |
| A�� | 10 g H2��10 g O2 | B�� | 5.6 L N2����״������22 g CO2 | ||
| C�� | 9 g H2O��0.5 mol Br2 | D�� | 224 mL H2��0.01 mol N2 |
| A�� | Fe2O3�ǻ�ԭ�� | B�� | Al����ԭ | ||
| C�� | Fe2O3������ԭ��Ӧ | D�� | ��Ӧ��ת��3������ |
| A�� | ��pH��ֽ�ⶨ��Һ��pH֮ǰ��Ҫ��������ˮʪ�� | |
| B�� | ����ˮʪ�����pH��ֽȥ�ⶨ�����pH�����ƫС | |
| C�� | ����ˮʪ�����pH��ֽȥ�ⶨ����������Һ��pH�����ƫС | |
| D�� | ������ˮ�����ԣ�����ֻ��ʹpH��ֽ��� |
| A�� | ԭ�������ĵ�������˵���������Ӷ����� | |
| B�� | ���ʵ���������˵���������Ӷ���ǿ | |
| C�� | �⻯����ȶ�����˵���������Ӷ����� | |
| D�� | �ǽ�������˵���������Ӷ���ǿ |
| A�� | ���� | B�� | ���ȴ��� | C�� | ������̼ | D�� | �������� |
| A�� | H2S��H2O��HF���ȶ������μ��� | B�� | RbOH��KOH��Mg��OH��2�ļ���������ǿ | ||
| C�� | Na+��Mg2+��Al3+�����������μ��� | D�� | H2SiO3��H2CO3��H2SO4����������ǿ |
| A�� | NO | B�� | SO2 | C�� | CO2 | D�� | Cl2 |