��Ŀ����
��ͼ�Ǹ��ֲ�ͬ�ܶȵ�����������Ӧ����ͬ�¶ȣ�ʱ��Ҫ��ԭ����ķֲ�ͼ���������ͼ�������ش��й����⡣

(1)������������ȷ���ǣ�����ţ�_______________��
A.ͼ�к�������������ܶȣ�������ܶ�Խ����Ũ��ԽС��
B.����ԭ�����¶���ͬʱ����ͬŨ�ȵ����ᱻ��ԭ�IJ��ﲻ�ǵ�һ�ģ�ֻ����ijŨ��ʱ����ij�ֲ���Ϊ�����ѡ�
C.�����Ũ��Խ��ԭ������NԪ�صͻ��ϼ۲���Խ��
D.�������ܶȴ���1.3g��cm-3ʱ����ԭ������ҪΪNO��NO2
(2)����ͼ��ѡ��������ҩƷ��֤��������1.36 g��cm-3���ᷴӦ�����������к���NO��N2��O2�����������ɿ��ƣ�����װ�ú̶�װ��ʡ�ԣ�

��֪����.NO+NO2+2OH-=2NO2-+H2O
��.�����£�NO2��N2O4��ϴ��ڣ���0��ʱ����ֻ����ɫ��N2O4Һ�������ڡ�
������������˳����������ӣ�����ӿڵı�ţ�Ϊ______________��
�ڷ�Ӧǰ��ͨ��N2��Ŀ����___________________��
��ȷ�������к���������__________________��
��װ��F��������________________��
������A�����Ļ��������NO2��NO������ֱ�ΪV1mL��V2mL��V1��V2����Ϊ0�����ұ���װ�������ܽ�������������ȫ���գ���װ����������Ҫ�������������Ϊ��________mL����ͬ״̬�£���
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�(NH4)2Fe(SO4)2•6H2O(Ī���Σ�dz��ɫ��ʽ��392)�ڶ��������г������궨������ء��ظ���ص���Һ�ı����ʣ���������ѧ�Լ���ҽҩ�Լ�����ұ�𡢵�Ƶȡ�
�ش��������⣺
��1��Ī�����ڿ����б����������ȶ���������¶���ڿ�����Ҳ����ʣ�����Ī�����Ƿ���ʵ��Լ���________��
��2��ȷ��ȡmg������Ī���Σ�����ƿ�м���20mLˮ����ܽ⣬��ij����K2Cr2O7��Һ�ζ����յ㣮�ظ�����3�Σ�����й��������£�
ʵ����� | ��ʼ����/mL | �յ����/mL |
I | 2.50 | 22.58 |
�� | 1.00 | 23.12 |
�� | 0.00 | 19.92 |
��K2Cr2O7��ҺӦ�÷���________ʽ�ζ����У�
��д���ζ������з�Ӧ�����ӷ���ʽ��________��
������K2Cr2O7��Һ�����ʵ���Ũ��Ϊ________mol/L(�ú�M�Ĵ���ʽ��ʾ)
��3��ij������ͨ��ʵ�����Ī���ξ������ʱ�ķֽ���
�ټ�ͬѧ������룺�ֽ���������N2��Fe2O3��SO3��H2O�������ʣ����Ƿ�ͬ�Ⲣ˵�����ɣ�________��
����ͬѧ�������ͼװ�ã�����Aװ���еĹ����Ϊ����ɫ�����������к���________��Cװ���к�ɫ��ȥ��˵����������к���________��Cװ�ú�Ӧ����β������װ��D��D��ʢ�е��Լ�������________(дһ�ּ���)��

�۱�ͬѧ����������װ��֤���ֽ�����к��а�����ֻ�����B��C�е��Լ����ɣ����������Լ�ΪB________��C________��
�ܶ�ͬѧ��ΪĪ���ηֽ���ܻ�����N2��SO3���������װ����ͼ2��ѡ���Ҫ��װ�ü���֤��������ȷ������˳�������������A��________��





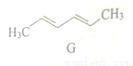
 _________________��
_________________�� CH3CH2Br
CH3CH2Br CH3CH2OH
CH3CH2OH