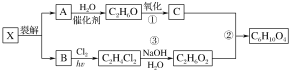
��Ŀ����
����Ŀ�����᳧�������·�������β����
(1)��Һ���շ�����![]() ��Һ����
��Һ����![]() ����
����![]() ����ȡ
����ȡ![]() ������
������![]() ��Һ��ȫ��Ӧʱת�Ƶ�����Ϊ
��Һ��ȫ��Ӧʱת�Ƶ�����Ϊ![]() ���÷�Ӧ�����ӷ���ʽ��______��
���÷�Ӧ�����ӷ���ʽ��______��
(2)�������շ���ԭ����![]() ��
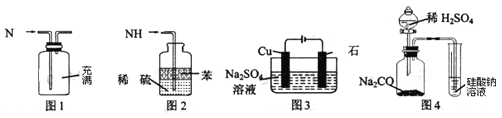
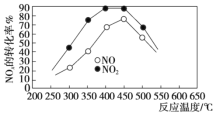
��![]() ��Ӧ�����������ʡ�ijͬѧ������ͼװ�úͲ���ģ�ҵ�ϵ��������ﴦ�����̣�
��Ӧ�����������ʡ�ijͬѧ������ͼװ�úͲ���ģ�ҵ�ϵ��������ﴦ�����̣�
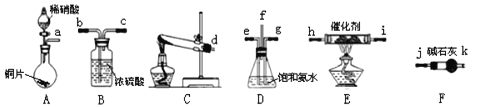
������������������ͼ����ͼ���ӳ�ģ��β������װ�ã�
![]()
�ٹܿ�f�ӹܿ�______��
��Dװ�õ�������______���ñ��Ͱ�ˮ�����ô�ˮ��Ŀ����______��
��Eװ���з�����Ӧ�Ļ�ѧ����ʽ��______��
���𰸡�2NO2+CO32=NO3+NO2+CO2 j ���������ٶȣ�ʹ�����Ͼ��� ��ֹ���� 4NH3+6NO![]() 5N2+6H2O��4xNH3+6NOx
5N2+6H2O��4xNH3+6NOx![]() (2x+3)N2+6xH2O
(2x+3)N2+6xH2O
��������
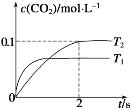
��1��9.2g���������������ʵ���n��NO2��=![]() =0.2mol��Na2CO3��Һ��ȫ��Ӧ����CO2ʱת�Ƶ���0.1mol����Ӧ��ֻ��NԪ�ػ��ϼ۱仯����N�Ļ��ϼ۷ֱ���+4�۱仯Ϊ+5�ۺ�+3�ۣ�
=0.2mol��Na2CO3��Һ��ȫ��Ӧ����CO2ʱת�Ƶ���0.1mol����Ӧ��ֻ��NԪ�ػ��ϼ۱仯����N�Ļ��ϼ۷ֱ���+4�۱仯Ϊ+5�ۺ�+3�ۣ�
��2��Aװ����ȡNO��Cװ����ȡ������Dװ�ð�����NO��ϣ�Fװ�ø��������壬Eװ�ð�����NO��Ӧ��Bװ�ô���������������������˳��Ϊa��e��d��g��f��j��k��i��h��b��c��
(1)![]() ��
��![]() ��Һ��ȫ��Ӧ����
��Һ��ȫ��Ӧ����![]() ʱת�Ƶ���
ʱת�Ƶ���![]() ����Ӧ��ֻ��NԪ�ػ��ϼ۱仯����N�Ļ��ϼ۷ֱ���
����Ӧ��ֻ��NԪ�ػ��ϼ۱仯����N�Ļ��ϼ۷ֱ���![]() �۱仯Ϊ
�۱仯Ϊ![]() �ۺ�
�ۺ�![]() �ۣ���Ӧ�����ӷ���ʽΪ2NO2+CO32=NO3+NO2+CO2��
�ۣ���Ӧ�����ӷ���ʽΪ2NO2+CO32=NO3+NO2+CO2��
�ʴ�Ϊ��2NO2+CO32=NO3+NO2+CO2��
(2)Aװ����ȡNO��Cװ����ȡ������Dװ�ð�����NO��ϣ�Fװ�ø��������壬Eװ�ð�����NO��Ӧ��Bװ�ô���������������������˳��Ϊa��e��d��g��f��j��k��i��h��b��c��
�ٹܿ�f�ӹܿ�j��
�ʴ�Ϊ��j��
��Dװ���еİ�ˮ�ܺͻӷ��������ᷴӦ�����ܵ��������ٶȣ�ʹ�����Ͼ��ȣ����Ͱ�ˮ�����ư����ܽ⣬���Է�ֹ������
�ʴ�Ϊ�����������ٶȣ�ʹ�����Ͼ��ȣ���ֹ������
��Eװ���а�����NO��Ӧ���ɵ�����ˮ��������Ӧ�Ļ�ѧ����ʽ��4NH3+6NO![]() 5N2+6H2O ��4xNH3+6NOx
5N2+6H2O ��4xNH3+6NOx![]() (2x+3)N2+6xH2O��
(2x+3)N2+6xH2O��
�ʴ�Ϊ��4NH3+6NO![]() 5N2+6H2O ��4xNH3+6NOx
5N2+6H2O ��4xNH3+6NOx![]() (2x+3)N2+6xH2O��
(2x+3)N2+6xH2O��

 ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�����Ŀ����ͼ�١��ڡ��ۡ��ܡ�����������(���ظ�ʹ��)��ѡ����ʵ�װ�ú�ҩƷ����ɵ�ʵ���ǣ� ��
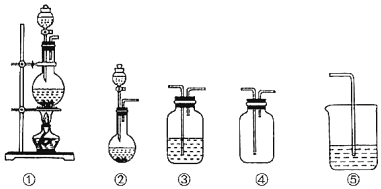
ѡ�� | ʵ��Ŀ�� | ʵ��װ�� | ʵ��ҩƷ |
A | �Ʊ����ռ�HCl���� | �٢ۢ� | Ũ���ᡢŨ���ᡢˮ |
B | �Ƚ����������������ǿ�� | �٢ۢ� | MnO2��Ũ���ᡢ����ʳ��ˮ���廯����Һ������������Һ |
C | ̽����ϩ�ļӳɷ�Ӧ | �٢� | ��ˮ�Ҵ���Ũ���ᡢ������Ȼ�̼��Һ |
D | ̽����������Ļ�ԭ�� | �ڢۢ� | �������ơ�30%�����ᡢ��ˮ������������Һ |
A.AB.BC.CD.D
����Ŀ����֪A(g)+B(g)![]() C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
�¶�/ �� | 700 | 800 | 830 | 1000 | 1200 |
ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
830��ʱ����һ��2 L���ܱ������г���0.2mol��A��0.8mol��B����Ӧ��ʼ4 s��A��ƽ����Ӧ����v(A)=0.005mol/(L��s)������˵����ȷ����
A��4 sʱc(B)Ϊ0.76mol/L
B��830����ƽ��ʱ��A��ת����Ϊ80%
C����Ӧ��ƽ��������¶ȣ�ƽ�������ƶ�
D��1200��ʱ��ӦC(g)+D(g��![]() A(g)+B(g)��ƽ�ⳣ����ֵΪ0.4
A(g)+B(g)��ƽ�ⳣ����ֵΪ0.4