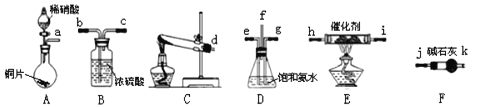
��Ŀ����
����Ŀ��2019��8��13���й���ѧ�Һϳ�������ȱ���յ��ľ�̬�������ε�һ��ְ����Ba2[Sn(OH)6][B(OH)4]2������˸û������LED���������о��������Ϊ�ⷢ�����ƺ�Ӧ���ṩһ���µ���Ч���ԡ�
(1)��̬Snԭ�Ӽ۵����Ų�ʽΪ_______����̬��ԭ�ӵļ۲�����Ų�ʽ���ܱ�ʾΪ2s22px22py2����Ϊ��Υ����_______ԭ��(����)��
(2)[B(OH)4]������ԭ�ӵ��ӻ��������Ϊ______����ԭ�ӵļ۲���ӶԻ���ģ����_____��[Sn(OH)6] 2���У�Sn��O֮��Ļ�ѧ����������_____��
a.���� b.���� c.��λ�� d.���Լ�
(3)������(NH3BH3)����Ϊ�����DZ�������ʹ������֮һ�������д�����λ����д��������Ľṹʽ_______����д��һ���백���黥Ϊ�ȵ�����ķ���_______(�ѧʽ)��
(4)��֪����(H3BO3)ΪһԪ���ᣬд���������һԪ�����Եĵ��뷽��ʽ________��
(5)̼�ᱵ��̼��þ�ֽ�õ��Ľ����������У��۵�ϸߵ���_______(�ѧʽ)����ԭ����___________��
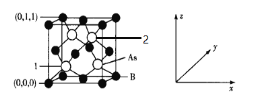
(6)�����ȵ��ʰ뵼����������黯��(BAs)�ľ����ṹ��ͼ��ʾ����2����ԭ�ӵ�����Ϊ______����֪�����ӵ�������ֵΪNA����������Asԭ�ӵ�Bԭ���������Ϊa pm����þ�����ܶ�Ϊ______g��cm��3(�г���a��NA�ļ���ʽ����)��
���𰸡�5s25p2 ���� sp3 ������ 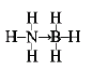 C2H6 H3BO3+H2O[B(OH)4]��+H+ a MgO BaO��MgO��Ϊ���Ӿ��壬þ���Ӱ뾶�ȱ����Ӱ뾶С��MgO�ľ����ܽϴ������۵�ϸ�
C2H6 H3BO3+H2O[B(OH)4]��+H+ a MgO BaO��MgO��Ϊ���Ӿ��壬þ���Ӱ뾶�ȱ����Ӱ뾶С��MgO�ľ����ܽϴ������۵�ϸ� ![]()
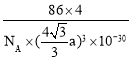
��������
����SnԪ�������ڱ��е�λ�ú�����Ԫ�صļ۵����Ų�ͨʽȷ����۵����Ų�ʽ�����ݼ۲���ӶԻ������۷���ԭ�ӵ��ӻ����ͺͼ۲���ӶԻ���ģ�ͣ�����B��O�ijɼ��ص��ж������γɵĻ�ѧ�����͡����ݹ��ۼ�����λ���ijɼ��ص��жϰ�����Ľṹʽ�����ݵȵ�����ĸ���Ѱ����ȵ����塣����Ӱ�쾧���ܵ����رȽ����Ӿ�����۵�ߵ͡����ݾ����ṹ�������ӵĿռ�ֲ��ص��ж���������������ݾ����ṹ�ص�ȷ����߳������ݾ�̯��ȷ�������е�ԭ�Ӹ�������һ�����ݾ�������������������ܶȡ�
(1)SnԪ��Ϊ50��Ԫ�أ�λ�ڵ�������IVA�壬���ݹ���ԭ���ɵû�̬Snԭ�Ӽ۵����Ų�ʽΪ5s25p2�����ݺ��ع��������Ų���ͬһ�ܼ��IJ�ͬ���ʱ����̬ԭ���еĵ����������ȵ���ռ��һ����������̬��ԭ�ӵļ۲�����Ų�ʽ��ʾΪ2s22px22py2Υ���˺��ع�����ȷ�ļ۲�����Ų�ʽӦ��ʾΪ2s22px22py12pz1���ʴ�Ϊ��5s25p2�����أ�
(2)[B(OH)4]������ԭ�������ĸ��ǻ�����۲���Ӷ���Ϊ4������ԭ�ӵ��ӻ��������Ϊsp3����ԭ�ӵ��������Ӷ���Ϊ2���µ��Ӷ���Ϊ2������ԭ�ӵļ۲���Ӷ���Ϊ4���۲���ӶԻ���ģ���������壻[Sn(OH)6] 2-����������Sn4+��OH-֮���γ���λ������λ����һ������Ĺ��ۼ�����Sn��O֮���γɵĻ�ѧ�������������Լ������������������ʴ�Ϊ��sp3�������壻a��
(3)��������Bԭ���ṩ�չ����NH3��Nԭ���ṩ1�Թµ��Ӷԣ��γ���λ����������(NH3BH3)�ĽṹʽΪ ���백���黥Ϊ�ȵ�����ķ���,������2��Cԭ�Ӵ���B��Nԭ��,���백���黥Ϊ�ȵ������һ�ַ���ΪC2H6���ʴ�Ϊ��
���백���黥Ϊ�ȵ�����ķ���,������2��Cԭ�Ӵ���B��Nԭ��,���백���黥Ϊ�ȵ������һ�ַ���ΪC2H6���ʴ�Ϊ�� ��C2H6��
��C2H6��
(4)����(H3BO3)ΪһԪ���ᣬ��ˮ�е���ʱ��������ˮ�������OH-�������ԣ�����������[B(OH)4]����H+�����뷽��ʽΪH3BO3+H2O[B(OH)4]��+H+���ʴ�Ϊ��H3BO3+H2O[B(OH)4]��+H+��
(5)̼�ᱵ��̼��þ�ֽ�õ��Ľ���������ֱ�ΪBaO��MgO�����߾�Ϊ���Ӿ��壬þ���Ӱ뾶�ȱ����Ӱ뾶С����MgO�ľ����ܽϴ�������۵�ϸߣ��ʴ�Ϊ��MgO��BaO��MgO��Ϊ���Ӿ��壬þ���Ӱ뾶�ȱ����Ӱ뾶С��MgO�ľ����ܽϴ������۵�ϸߣ�
(6)�۲쾧���ṹ��֪��2����ԭ�ӵ�����Ϊ![]() ���۲�ṹ��Asԭ�ӵ�Bԭ��������������������������Խ��ߵ�
���۲�ṹ��Asԭ�ӵ�Bԭ��������������������������Խ��ߵ�![]() ���辧���ı߳�Ϊd pm����Asԭ�ӵ�Bԭ���������Ϊ
���辧���ı߳�Ϊd pm����Asԭ�ӵ�Bԭ���������Ϊ![]() ����
����![]() ��Asλ�ھ������ڣ������е�Asԭ����ĿΪ4��Bλ�ڶ�������ģ��������е�Bԭ����ĿΪ
��Asλ�ھ������ڣ������е�Asԭ����ĿΪ4��Bλ�ڶ�������ģ��������е�Bԭ����ĿΪ![]() ����þ�����ܶ�Ϊ
����þ�����ܶ�Ϊ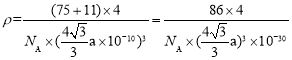 ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��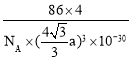 ��
��

 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�����Ŀ����ҵ����CO��H2�ϳɼ״���CO(g)+2H2(s)![]() CH3OH(g) ��H=��90.8kJ/mol��300��ʱ�����ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������
CH3OH(g) ��H=��90.8kJ/mol��300��ʱ�����ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й���������
���� | �� | �� | �� | |
��Ӧ��Ͷ���� | 1 molCO��2molH2 | 1mol CH3OH | 2mol CH3OH | |
ƽ��ʱ���� | CH3OH��Ũ��(mol/L) | c1 | c2 | c3 |
��Ӧ�������仯 | a kJ | b kJ | c kJ | |
��ϵѹǿ��Pa�� | P1 | P2 | P3 | |
��Ӧ��ת���� | a1 | a2 | a3 | |
����˵����ȷ����
A.2c1>c3B.a+b<90.8C.2P2<P3D.a1+a3<1
����Ŀ��![]() ʱ�������Ϊ
ʱ�������Ϊ![]() �����������ܱ������з������淴Ӧ��
�����������ܱ������з������淴Ӧ��![]()
![]() ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������������������ȷ����
ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������������������ȷ����![]()
������� | ��ʼʱ���������ʵ��� | �ﵽƽ��ʱ��ϵ�����ı仯 | ||
A | B | C | ||
| 2 | 1 | 0 |
|
|
|
|
| |
A.����![]() ��
��![]() �е�ƽ�ⳣ����Ϊ36
�е�ƽ�ⳣ����Ϊ36
B.������![]() ��ͨ�뺤����ƽ��ʱA��ת���ʲ���
��ͨ�뺤����ƽ��ʱA��ת���ʲ���
C.����![]() �дﵽƽ��ʱ�ų�������Ϊ/span>
�дﵽƽ��ʱ�ų�������Ϊ/span>![]() QkJ���ﵽƽ��ʱ������������C�����ʵ���Ũ�Ⱦ�Ϊ
QkJ���ﵽƽ��ʱ������������C�����ʵ���Ũ�Ⱦ�Ϊ![]()
![]()
D.�����������䣬������![]() ���ֺ��ݾ��ȣ���ﵽƽ��ʱC���������С��
���ֺ��ݾ��ȣ���ﵽƽ��ʱC���������С��![]()