��Ŀ����
���÷�ӦI2(s)+Cl2(g)=2ICl(l)��ʵ���ҿ�������ͼ��ʾװ�ã����ȡ��г���������ȥ����ȡ����IC1��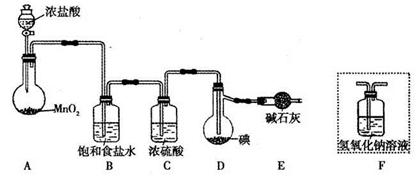
��֪��ICl���۵�Ϊ13.9�棬�е�Ϊ97.4�棬��ˮ�⣬���ܷ�����Ӧ��
ICl(l)+Cl2(g)=2ICl3(l)
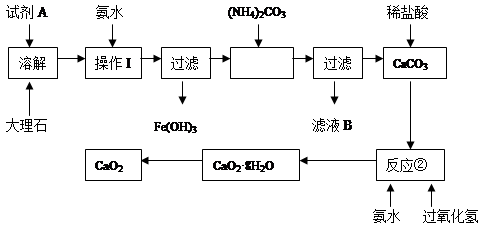
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��____________��
��2��װ��B��������______��������װ��F����װ��E��������____________��
��3�����Ƶõ�ICl����������ICl3���ʣ��ᴿ�ķ�����______ (���ţ���
| A������ | B�������ᾧ | C������ | D����Һ |
i��

ii��ICl+KI=I2+KCl
iii��I2+2Na2S2O3=2NaI+Na2S4O6
ʵ��1����0.500g����֬��Ʒ����10mL���Ȼ�̼����20mLijICl�ı�������Һ������������ַ�Ӧ��������KI��Һ�����ɵĵⵥ����a mol?L��1��Na2S2O3������Һ�ζ�����ƽ��ʵ�飬������ĵ�Na2S2O3��Һ��ƽ�����ΪV1mL��
ʵ��2���հ�ʵ�飩��������֬��Ʒ�������������衢�����Լ���������ʵ��1��ȫ��ͬ��������ĵ�Na2S2O3��Һ��ƽ�����ΪV2mL��
�ٵζ������п���______��ָʾ����
�ڵζ���������Ҫ����������ᵼ��V1______���ƫ��ƫС������
��0.500g����֬��Ʒ�����ĵ�ICl�����ʵ���Ϊ______mol���ɴ����ݾ����㼴����ø���֬�IJ����Ͷȡ�
��16�֣�
��1��MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O��3�֣�
MnCl2+Cl2��+2H2O��3�֣�
��2����ȥ�����е��Ȼ������ʣ�2�֣�
װ��F�е�ˮ���������װ��D�У�ʹIClˮ�⣨�����������𰸣���2�֣�
��3��C��2�֣�
��4���ٵ�����Һ��2�֣�
��ƫС��2�֣�
��5a(V2��V1)��10��4��3�֣�
���������������1������ʱ��������������Ũ���ᣬ��ӦʽΪMnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O����2����װ��A���ݳ���������Ҫ�ɷ����������Ȼ����ˮ����������ʳ��ˮ���ܽ��Ȼ��⣬�����ܽ�������Ũ��������ˮ����װ��B�������dz�ȥ�����е��Ȼ������ʣ�����F����E��װ��F�е�ˮ���������װ��D�У�ʹIClˮ��[��ICl(l)+Cl2(g)=2ICl3(l)]�������ò�Ʒ��������3��������֪��Ϣ��֪��ICl(l)��ICl3(l)��ΪҺ�壬�һ��ܡ��е����Բ�ͬ���ɻ������롢�ᴿ�ķ�����֪���Ӷ�IJ�Ʒ�ᴿ�ķ���Ϊ���������ʺϷ����Һ�����ܵĻ��������ᾧ�ʺϻ��ܻ��������һ���ܽ�ȵ����ʣ���Һ�ʺϻ������ܵĻ�����4���������ڵ�����Һ��I2��������ζ������п��õ�����ָʾ�����ڵζ���������Ҫ������ʹI2��Na2S2O3��ַ�Ӧ��ʹ��Һ��ɫ�Ұ�����ڲ��ָ�Ϊ��ɫ������Na2S2O3��Һ���㣬V1ƫС��ʵ��1��n(Na2S2O3)=c?V=a?V1��10��3mol���ɷ�Ӧiii�з�Ӧ���ϵ��֮�ȵ������ʵ���֮�ȣ���n(I2)= n(Na2S2O3)/2= 5a?V1��10��4mol���ɷ�Ӧii�з�Ӧ���ϵ��֮�ȵ������ʵ���֮�ȣ���n(ICl)= n(I2)= 5a?V1��10��4mol��ʵ��2��n(Na2S2O3)=c?V=a?V2��10��3mol����n(I2)= n(Na2S2O3)/2= 5a?V2��10��4mol��n(ICl)= n(I2)= 5a?V2��10��4mol���ȽϿ�֪��ʵ��1�б�C=C�����ĵ�n(ICl)= 5a?V2��10��4mol��5a?V1��10��4mol=5a(V2��V1)��10��4mol��
MnCl2+Cl2��+2H2O����2����װ��A���ݳ���������Ҫ�ɷ����������Ȼ����ˮ����������ʳ��ˮ���ܽ��Ȼ��⣬�����ܽ�������Ũ��������ˮ����װ��B�������dz�ȥ�����е��Ȼ������ʣ�����F����E��װ��F�е�ˮ���������װ��D�У�ʹIClˮ��[��ICl(l)+Cl2(g)=2ICl3(l)]�������ò�Ʒ��������3��������֪��Ϣ��֪��ICl(l)��ICl3(l)��ΪҺ�壬�һ��ܡ��е����Բ�ͬ���ɻ������롢�ᴿ�ķ�����֪���Ӷ�IJ�Ʒ�ᴿ�ķ���Ϊ���������ʺϷ����Һ�����ܵĻ��������ᾧ�ʺϻ��ܻ��������һ���ܽ�ȵ����ʣ���Һ�ʺϻ������ܵĻ�����4���������ڵ�����Һ��I2��������ζ������п��õ�����ָʾ�����ڵζ���������Ҫ������ʹI2��Na2S2O3��ַ�Ӧ��ʹ��Һ��ɫ�Ұ�����ڲ��ָ�Ϊ��ɫ������Na2S2O3��Һ���㣬V1ƫС��ʵ��1��n(Na2S2O3)=c?V=a?V1��10��3mol���ɷ�Ӧiii�з�Ӧ���ϵ��֮�ȵ������ʵ���֮�ȣ���n(I2)= n(Na2S2O3)/2= 5a?V1��10��4mol���ɷ�Ӧii�з�Ӧ���ϵ��֮�ȵ������ʵ���֮�ȣ���n(ICl)= n(I2)= 5a?V1��10��4mol��ʵ��2��n(Na2S2O3)=c?V=a?V2��10��3mol����n(I2)= n(Na2S2O3)/2= 5a?V2��10��4mol��n(ICl)= n(I2)= 5a?V2��10��4mol���ȽϿ�֪��ʵ��1�б�C=C�����ĵ�n(ICl)= 5a?V2��10��4mol��5a?V1��10��4mol=5a(V2��V1)��10��4mol��
���㣺�����������Ʒ��������ķ�����ᴿ��������ԭ��Ӧ�ζ�����ϢǨ�ơ����ʵ����ڻ�ѧ����ʽ�����е�Ӧ�õ����֪ʶ��

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д������������ʵķ�����ȷ����(����)
| A���������������ڶ���̼�� |
| B��ˮ���������ڴ��������IJ���ƿ�� |
| C�������������ھƾ��� |
| D��������������ú���� |
��1������ʵ��������ʵ����ʵ����������ȷ���� ������ţ���
| A����������ƽ����17.55g�Ȼ��ƾ��� |
| B��̼������Һ�����ڴ����������Լ�ƿ |
| C���ø����pH��ֽ�ⶨ������ˮ��pH |
| D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ�� |
F����ȥCO2�����л��е�����HCl�����Խ�����ͨ�뱥��̼��������Һ
��2����ͼΪ��ѧ��ѧʵ���г�����ʵ��װ��
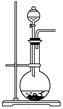


A B C
ʵ���ҳ���װ��A�Ʊ��±������壬�뽫��Һ©����Բ����ƿ��Ӧװ�Ļ�ѧ�Լ���д������
| ���� | O2 | Cl2 | NH3 |
| ��Һ©�����Լ� | | | Ũ��ˮ |
| Բ����ƿ���Լ� | | KMnO4 | |
����Bװ����Һ�ռ����壬����Ӧ�Ӹ�װ��________(����ҡ�)�ܿڵ������������ø�װ���ռ�Cl2���Լ�ƿ��ʢ�ŵ��Լ�Ϊ ��
Cװ�����ڴ�����������Ի�������Ⱦ�������ø�װ������Cl2����ʱ�ձ��з�����Ӧ�����ӷ���ʽΪ ��������װ���н���ʢ��ϡ���ᣬͨ�����ʺ����հ�����ԭ���� �������ձ��ж����ټ���һ��Һ̬�л�����ɰ�ȫ���հ����������л���Ϊ ��
����ҩƷ��װ�ú������������Ӧʵ�����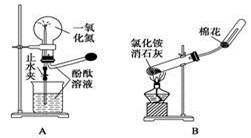
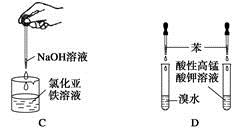
| A����Ȫʵ�� |
| B��ʵ������ȡ���ռ����� |
| C���Ʊ����������� |
| D����֤�����Ƿ���̼̼˫�� |
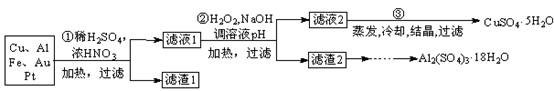
 12Na2CrO4+3Fe2O3+7KCl+12H2O
12Na2CrO4+3Fe2O3+7KCl+12H2O