��Ŀ����
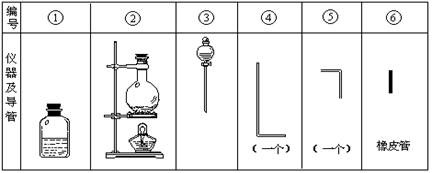
��1������ʵ��������ʵ����ʵ����������ȷ���� ������ţ���
| A����������ƽ����17.55g�Ȼ��ƾ��� |
| B��̼������Һ�����ڴ����������Լ�ƿ |
| C���ø����pH��ֽ�ⶨ������ˮ��pH |
| D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ�� |
F����ȥCO2�����л��е�����HCl�����Խ�����ͨ�뱥��̼��������Һ
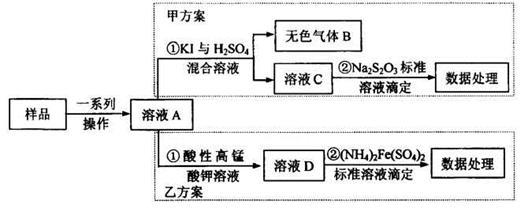
��2����ͼΪ��ѧ��ѧʵ���г�����ʵ��װ��


A B C
ʵ���ҳ���װ��A�Ʊ��±������壬�뽫��Һ©����Բ����ƿ��Ӧװ�Ļ�ѧ�Լ���д������
| ���� | O2 | Cl2 | NH3 |
| ��Һ©�����Լ� | | | Ũ��ˮ |
| Բ����ƿ���Լ� | | KMnO4 | |
����Bװ����Һ�ռ����壬����Ӧ�Ӹ�װ��________(����ҡ�)�ܿڵ������������ø�װ���ռ�Cl2���Լ�ƿ��ʢ�ŵ��Լ�Ϊ ��
Cװ�����ڴ�����������Ի�������Ⱦ�������ø�װ������Cl2����ʱ�ձ��з�����Ӧ�����ӷ���ʽΪ ��������װ���н���ʢ��ϡ���ᣬͨ�����ʺ����հ�����ԭ���� �������ձ��ж����ټ���һ��Һ̬�л�����ɰ�ȫ���հ����������л���Ϊ ��
��1��ABCE��4�� ÿ��ѡ1������©1������1�֣�
��2���٣���4�֣�
���� ����ʳ��ˮ���� O2 Cl2 NH3 ��Һ©�����Լ� H2O2����H2O�� Ũ���� Ũ��ˮ Բ����ƿ���Լ� MnO2����Na2O2�� KMnO4 NaOH����CaO��
��Cl2��2OH��=ClO����Cl����H2O ������������ˮ��ϡ���ᣩ����������
CCl4(�����Ȼ�̼)
���������������1��A����������ƽ����ֻ�ܾ�ȷ��0.1g������B��̼������Һ�ʼ��Ի�Ͳ������еĶ������跴Ӧ������ճ�Ե����ʹ����ƣ���ʹ�������Ͳ���ƿճ��һ�𣬴���C���ø����pH��ֽ�ⶨ������ˮ��pH��������ˮ��Ư���Ի�ʹ��ֽ������ɫ���۲죬����D��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�Ũ��ƫ����ȷ��E������е�����ˮ���������뱥��FeCl3��Һֱ�����ɺ��ɫҺ�壬������ȡFe(OH)3���� �ij��÷���������F����ȥCO2�����л��е�����HCl�����Խ�����ͨ�뱥��̼��������Һ����ȷ����ѡΪABCE����2������ͼ�ֱ����Ʊ����������ԭ��
��Ҫ��Bװ����Һ�ռ����壬����Ӧ�Ӹ�װ��_�ҹܿڵ������������ܰ�Һ���ų����ռ�Cl2���Լ�ƿ��ʢ�ŵ��Լ�Ϊ����ʳ��ˮ����һ�����ȥ�Ȼ��⣬��һ�����������ܽ��ԡ����������������Ƶķ�Ӧ���ӷ���ʽΪCl2��2OH��=ClO����Cl����H2O��������װ���н���ʢ��ϡ���ᣬͨ�����ʺ����հ�����ԭ���� ������������ˮ��ϡ���ᣩ�����������������ձ��ж����ټ���һ��Һ̬�л�����ɰ�ȫ���հ����������л���ΪCCl4(�����Ȼ�̼)�����ʵ��ܶȱ�ˮ���������ܽ������С����� O2 Cl2 NH3 ��Һ©�����Լ� H2O2����H2O�� Ũ���� Ũ��ˮ Բ����ƿ���Լ� MnO2����Na2O2�� KMnO4 NaOH����CaO��
���㣺���鳣��ʵ�����������ʵ�����Ʊ�����Ļ���ԭ����

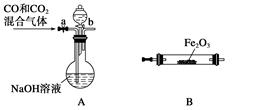
���÷�ӦI2(s)+Cl2(g)=2ICl(l)��ʵ���ҿ�������ͼ��ʾװ�ã����ȡ��г���������ȥ����ȡ����IC1��
��֪��ICl���۵�Ϊ13.9�棬�е�Ϊ97.4�棬��ˮ�⣬���ܷ�����Ӧ��
ICl(l)+Cl2(g)=2ICl3(l)
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��____________��
��2��װ��B��������______��������װ��F����װ��E��������____________��
��3�����Ƶõ�ICl����������ICl3���ʣ��ᴿ�ķ�����______ (���ţ���
| A������ | B�������ᾧ | C������ | D����Һ |
i��

ii��ICl+KI=I2+KCl
iii��I2+2Na2S2O3=2NaI+Na2S4O6
ʵ��1����0.500g����֬��Ʒ����10mL���Ȼ�̼����20mLijICl�ı�������Һ������������ַ�Ӧ��������KI��Һ�����ɵĵⵥ����a mol?L��1��Na2S2O3������Һ�ζ�����ƽ��ʵ�飬������ĵ�Na2S2O3��Һ��ƽ�����ΪV1mL��
ʵ��2���հ�ʵ�飩��������֬��Ʒ�������������衢�����Լ���������ʵ��1��ȫ��ͬ��������ĵ�Na2S2O3��Һ��ƽ�����ΪV2mL��
�ٵζ������п���______��ָʾ����
�ڵζ���������Ҫ����������ᵼ��V1______���ƫ��ƫС������
��0.500g����֬��Ʒ�����ĵ�ICl�����ʵ���Ϊ______mol���ɴ����ݾ����㼴����ø���֬�IJ����Ͷȡ�
�к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
(1)����ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��_________ _������֮�⣬װ���е�һ�����Դ����� ��
(2)Ϊ��֤��ʵ��ɹ���ͬѧ��ȡ�������ʩ����ͼ����ֽ������������________ ___��
(3)������60mL 0.25mol��L-1 H2SO4��50mL 0.55mol��L-1 NaOH��Һ���з�Ӧ������ʵ����ȣ����ų������� �����ȡ���������ȡ�������ʵ���������ȷ���������к��� ���ȡ�������ȡ���
(4)����NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز�������������
B����������������
C��һ��Ѹ�ٵ���
(5)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ���
B���ҿ�ӲֽƬ�ò���������
C����������ձ�
D���������¶ȼ��ϵĻ��β���������ؽ���
(6)ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к��Ȧ�H��______ ____ ( ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)____ ____��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
ijNa2CO3��Ʒ�л���һ������Na2SO4 (��������ᾧˮ����ij��ѧ��ȤС��������ַ����ⶨ����Ʒ��Na2CO3�������������Իش��������⡣
����һ���������з������Na2CO3���������Ĝy��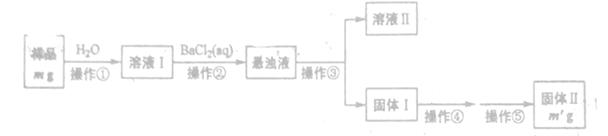
(1)�����ۺܵ͢����Ʒֱ�Ϊ_______��
(2)���������١����У�ʹ�õ�����������______(��������)��
(3)�жϲ����ڷ���ɵķ�����______
��������������ͼʵ��װ�ã��г�������ʡ�ԣ�.ѡ�������Լ�: a.Ũ����b.����NaHCO3��ҺC.6mol/L����D.2mol/L����, e.��ʯ��f. ��ˮCaCl2,�y����Ʒ��Na2CO3,������������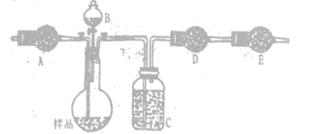
(4)��д���пո�
| ���� | �Լ� | ������Լ���Ŀ�� |
| A | | �������ʱϴȥCO2 |
| B | | ʹ��Ʒ��ַ�Ӧ�ų����� |
| C | a | |
| D | e | �������CO2 |
| E | e | |




 Mg��OH��2
Mg��OH��2 MgCl2��Һ��MgCl2��6H2O��MgCl2
MgCl2��Һ��MgCl2��6H2O��MgCl2 Mg
Mg