��Ŀ����
��֪ij�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��ΪK�����¶�����20 mL 0.1 mol��L��1 CH3COOH��Һ����μ���0.1 mol��L��1 NaOH��Һ����pH�仯������ͼ��ʾ(�����¶ȱ仯)������˵���в���ȷ����
| A��a��ʱ��CH3COOH�ĵ������1% |
| B��b��ʱ����Һ��c(CH3COO��)��c(Na��) |
| C��c���ʾCH3COOH��NaOHǡ�÷�Ӧ��ȫ |
D��b��d���ʾ����Һ�� ������K ������K |
C
�������������C����Һ��p H=7������CH3COOH��NaOHǡ�÷�Ӧ��ȫʱ������ΪCH3COO Na��p H>7,����
���㣺������ʡ�

��ϰ��ϵ�д�
�����Ŀ
���е������Һ���й�������ȷ����
| A��ͬŨ�ȡ�ͬ�����ǿ����ǿ����Һ��Ϻ���Һ��pH=7 |
| B���ں���BaSO4��������Һ�м���K2SO4���壬c(Ba2+)���� |
| C����l mol KOH����Һ��l mol CO2��ȫ��Ӧ����Һ��c(K+)��c(HCO3-) |
| D����(NH4)2SO4��Һ�м�������H2SO4����ʹc(NH4+)=2c(SO42-) |
����ˮ������ӷ���ʽ��ȷ���Ǹ߿���Դ��
A��HS����H2O S2-��H3O+ S2-��H3O+ |
B��Fe3����3H2O Fe(OH)3����3H�� Fe(OH)3����3H�� |
C��CO32����2H2O H2CO3��2OH�� H2CO3��2OH�� |
D��NH4����H2O NH3��H2O��H�� NH3��H2O��H�� |
�����£���1 mL0.l mol/L�� H2SO4��ˮϡ���Ƴ�2L��Һ���ڴ���Һ����ˮ��������� H��Ũ�Ƚӽ���
| A��1.0��10��4 mol/L | B��1.0��10�� 8 mol / L |
| C��1.0��10��10 mol/L | D��1.0��10��11mol/L |
��Ӱ��ˮ�ĵ���ƽ�⣬��ʹ��Һ�е�c(H��)��c(OH��)�Ĵ�ʩ��
| A����ˮ��ͨ��SO2 | B����ˮ������� |
| C����ˮ��Ͷ��һС������� | D����ˮ�м���NaCl |
�����£�pH��13��ǿ����Һ��pH��2��ǿ����Һ��ϣ����û��Һ��pH��11����ǿ����ǿ����������
| A��11��1 | B��9��1 | C��1��11 | D��1��9 |
�����£��йش�����Һ�������д������(˫ѡ) (����)��
| A��pH��5.6��CH3COOH��CH3COONa�����Һ�У�c(Na��)<c(CH3COO��) |
B����pH��3�Ĵ���ϡ��ΪpH��4�Ĺ����У�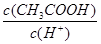 ��ֵ���� ��ֵ���� |
| C��Ũ�Ⱦ�Ϊ0.1 mol��L��1��CH3COOH��CH3COONa��Һ�������Ϻ�c(CH3COO��)��c(CH3COOH)��2c(Na��) |
| D��a mL pH��3�Ĵ�����Һ��b mL pH��11��NaOH��Һǡ����ȫ�к�ʱ��a��b |
