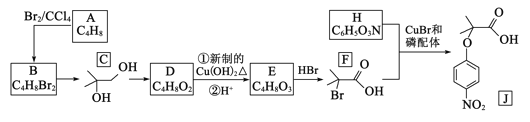
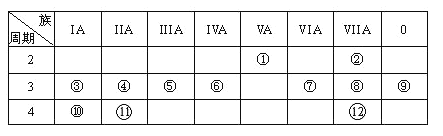
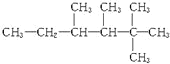��Ŀ����
����Ŀ��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
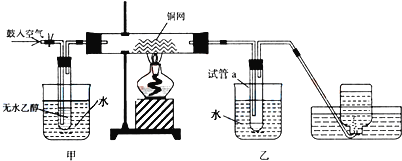
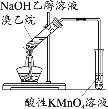
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ��___��__��
��2���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��__��Ӧ��
��3����������ˮԡ���ò���ͬ����������__���ҵ�������__��
��4����Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������__������ƿ���ռ������������Ҫ�ɷ���___����д���ƣ�
��5�����Թ�a���ռ�����Һ����ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����__����ȥ�����ʣ������ڻ��Һ�м���___(��д��ĸ)��
a.�Ȼ�����Һ b.�� c.̼��������Һ d.���Ȼ�̼
Ȼ����ͨ��___(��ʵ���������)���ɳ�ȥ��
���𰸡�2Cu+O2![]() 2CuO CH3CH2OH+CuO
2CuO CH3CH2OH+CuO![]() CH3CHO+Cu+H2O ���� �����Ҵ��������Ҵ��Ļӷ� ��ȴ��������ȩ���ռ� ��ȩ���Ҵ���ˮ ���� ���� C ����
CH3CHO+Cu+H2O ���� �����Ҵ��������Ҵ��Ļӷ� ��ȴ��������ȩ���ռ� ��ȩ���Ҵ���ˮ ���� ���� C ����
��������
��1���Ҵ��Ĵ�����ʵ����Cu��O2��Ӧ���ɺ�ɫCuO��CuO���Ҵ���Ӧ����Cu����ȩ��������Cu��O2��Ӧ�������ֺ�ڽ�������
��2���������ܷ�Ӧ��˵���÷�Ӧ���ȣ�
��3�����в����Ҵ����������з�Ӧ����Ӧ�����ɵ���ȩ������������
��4�����Ⱥ�δ��Ӧ�ķ�Ӧ���Ҵ���O2�������Ҵ��������б�������
��5�����Թ�a���ռ�����Һ����ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ�������ᣬֻ����Ϊ���ᣬ�ݴ˻ش�
��1���ɷ�����֪��ͭ�����ֺ�ڽ��������������������Ӧ��2Cu+O2![]() 2CuO ��CH3CH2OH+CuO
2CuO ��CH3CH2OH+CuO![]() CH3CHO+Cu+H2O���ʴ�Ϊ��2Cu+O2
CH3CHO+Cu+H2O���ʴ�Ϊ��2Cu+O2![]() 2CuO��CH3CH2OH+CuO
2CuO��CH3CH2OH+CuO![]() CH3CHO+Cu+H2O��
CH3CHO+Cu+H2O��
��2���������ܷ�Ӧ��˵���÷�Ӧ���ȣ���Ϊ�����ȣ�
��3����������ʹ�Ҵ��ӷ��ģ�ӦΪ��ˮԡ��������ʹ��ȩ�����ģ�ӦΪ��ˮԡ���ˮԡ���ʴ�Ϊ�������Ҵ��������Ҵ��Ļӷ�����ȴ��������ȩ���ռ���
��4�����Ⱥ�δ��Ӧ�ķ�Ӧ���Ҵ���O2�������Ҵ��������б�����������ƿ���ռ��������뷴Ӧ�ĵ������ʴ�Ϊ����ȩ���Ҵ���ˮ��������
��5�����Թ�a���ռ�����Һ����ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ�������ᣬ��������һ������ȩ�����������ᣬ���ȥ���ᣬ����NaHCO3���䷴Ӧ��Ȼ���������ᴿ���ʴ�Ϊ�����C������

 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�����Ŀ������ʵ���У��ܴﵽ��Ӧʵ��Ŀ�ĵ���
|
|
|
|
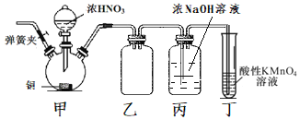
A���Ʊ����ռ��������� | B��֤���Ȼ����ܽ�ȴ������� | C����֤���������ȥ��������ϩ | D���ƶ�S��C��Si�ķǽ�����ǿ�� |
A.AB.BC.CD.D
����Ŀ���������ȣ�ClO2���㷺Ӧ����ֽ��Ư�ס�ɱ��������ˮ��������������ҵ�����ü״���ԭNaClO3�ķ����Ʊ�ClO2�������������£�
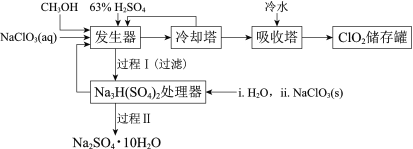
��֪��a�����������Ʊ�ClO2�ķ�Ӧ��12NaClO3+8H2SO4+3CH3OH= 12ClO2��+3HCOOH+4Na3H(SO4)2��+9H2O
b��������ʵ��۷е㣺
���� | CH3OH | HCOOH | ClO2 |
�۵�/�� | ��97 | 9 | ��59 |
�е�/�� | 65 | 101 | 11 |
(1)ClO2������ֽ��Ư�ס�ɱ���������������______�ԡ�
(2)��ȴ�����ڷ���ClO2������CH3OH��Ӧ���Ƶ�����¶�Ϊ______������ĸ����
A��0~10�� B��20~30�� C��60~70��
(3)�����̢���̢���Ի��â����Na2SO4��10H2O����ʹ����ԭ��ѭ�����á�
��֪��Na2SO4��10H2O��Na2SO4���ܽ����������ͼ��
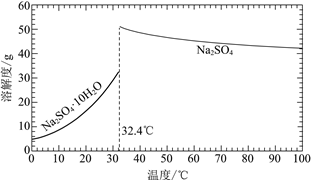
��Na3H(SO4)2�������л��â��ʱ�����NaClO3���壬��â���ܽ�ƽ��ĽǶȽ�����ԭ��______��
�ڽ��Na2SO4��10H2O��Na2SO4���ܽ�����ߣ����̢�IJ����ǣ���32.4�����������______��
��Na3H(SO4)2����������Һ�п���ѭ�����õ�ԭ����NaClO3��______��