��Ŀ����
����Ŀ������ѧ����ѡ��3�����ʽṹ�����ʡ�
����Ͻ������ý�����Ͻ������γ��⻯������ⴢ��������MgH2��LaNi5��MmNiMn��Mm�������ϡ�������Ǽ��ೣ���Ĵ�����ϡ����ڣ��й��ƴ�������о����֣������ı��渲��ʯīϩ����ͼ��ʾ�������Դ�����Ӵ�������ͷ����������ʡ���֪��ʯīϩ��һ���ɵ���̼ԭ����ɵ�ƽ��ṹ���������õĵ��硢�����ԡ��ش��������⣺
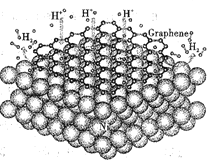
��1��Mn�ļ۵����Ų�ʽΪ_______________���ݴ˿��ж�MnԪ����Ԫ�����ڱ��д���_________����
��2����ʯīϩ��̼ԭ�ӵ��ӻ���ʽΪ_______________���ݴ�˵��ʯīϩ�������õ����Ե�ԭ����_____________________��
��Si����C���ڵ�ͬ����Ԫ�أ���ԭ�ӽṹ������Ԥ�⣬Si�ܷ��γ�����ʯīϩ���ȶ��ṹ��
________________________��
��3��̼�������γ����顢��ϩ����Ȳ�ȶ����л����֪������ͬ�����£���ϩ����Ȳ���������ӳɣ� �����������顣
������Ķ��������____________�����������������������Է��ӡ�
���ӹ��ۼ����γɹ���˵����ϩ��������õ�ԭ��____________________��
��C��C,C![]() C��C
C��C![]() C�ļ���֮�����п���Ϊ___________�����������
C�ļ���֮�����п���Ϊ___________�����������
A��1.00��2.17��4.90
B��1.00��1.77��2.34
C��1.00��2.00��3.00
��4��Ni��������֮���������Ϊ________��Ni�ľ���������ͭ����������ͬ����Ni�����뾶��rNi���г�Ni����Ŀռ�ռ���ʵı���ʽ��__________________��
���𰸡���1��3d54s2��d
��2����sp2�ӻ���ÿ��̼ԭ����һ��δ�����ӻ���p�����ֱ��̼ԭ��ƽ�棬����p����ƽ���ص���ʹp����еĵ��ӿ���������ƽ�����˶���������Si��ԭ�Ӱ뾶��C�����γɵ������ϳ���p��p����ص���С�������γ��ȶ���![]() ��
��
��3����������ϩ���γ��Ħм����������ι�������������B
��4����������
��������
�����������1��MnԪ�ص�ԭ������Ϊ25��Mn�ļ۵����Ų�ʽΪ3d54s2���ݴ˿��ж�MnԪ����Ԫ�����ڱ��д���d����
��2����ʯīϩ��ͬ��̼ԭ������ƽ��ṹ������C���ӻ���ʽΪsp2�ӻ���ÿ��̼ԭ����һ��δ�����ӻ���p�����ֱ��̼ԭ��ƽ�棬����p����ƽ���ص���ʹp����еĵ��ӿ���������ƽ�����˶�������ʯīϩ�������õ�������
��Si����C���ڵ�ͬ����Ԫ�أ���������Si��ԭ�Ӱ뾶��C�����γɵ������ϳ���p��p����ص���С�������γ��ȶ���![]() ��������Si�����γ�����ʯīϩ���ȶ��ṹ��
��������Si�����γ�����ʯīϩ���ȶ��ṹ��
��3��̼�������γ����顢��ϩ����Ȳ�ȶ����л����֪������ͬ�����£���ϩ����Ȳ���������ӳɣ� �����������顣
�� ����̼ԭ����ָͬʱ��4����ͬ��ԭ�ӻ�ԭ����������̼ԭ�ӣ���������Ķ�������������Է��ӡ��ʴ�Ϊ������
�� ������ϩ�е�̼̼˫��������һ�������м������������ι�����������������ϩ��������á��ʴ�Ϊ����ϩ���γ��Ħм����������ι�����������
�� �������ķ�����֪��C=C�ļ���С��C-C�Ķ���������C-C��C=C��C��C�ļ���֮�����п���ΪB���ʴ�Ϊ��B��
��4��NiΪ�������壬��������֮���������Ϊ��������Ni�ľ���������ͭ����������ͬ��Ϊ�����������ܶѻ���������ԭ������ά�ռ��������ò��ȡABCABC���ѻ����ռ�������Ϊ�ɱ�ʾΪ ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����һ����������ʵ�ֶ��Ļ�ѧʵ��̽����ij����С��ѧ������ͼ15��ʾ�����������ʡ�Լгֺ;���װ�������������ʵ�飬�ش��������⣺
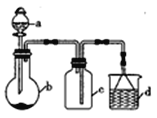
��1������a������Ϊ______________������a��ʹ��ǰҪ________________��
��2����װ�ÿ�����ijЩ�������ȡ���ռ���β�������b�ã��±���3��ʵ�����Ʒ�����������_________________���������
ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
A | ϡ���� | Cu | NO | H2O |
B | ϡH2SO4 | CaCO3 | CO2 | NaOH��Һ |
C | Ũ��ˮ | NaOH���� | NH3 | H2O |
��3�������������Ӵ�ʱ��������̣���д����Ӧ�Ļ�ѧ����ʽ��_______________________��
��4������װ����a�е�����ΪŨ���ᣬb�е�����ΪCuƬ���ڼ���������Ҳ����ȡSO2��
������װ�ÿ�����SO2β�������������г���������ȥ��_____________�����������
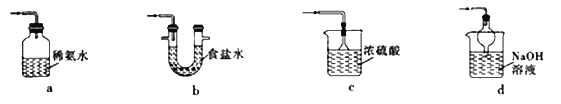
��Fe2��SO4��3��ҺҲ�����ն����SO2����.д��SO2��Fe2��SO4��3��Һ��Ӧ�����ӷ���ʽ��_______________________��
��ijС���÷�Ӧ������CuSO4��Һ����ȡ�������ⶨ���õ�����CuSO4��xH2O���нᾧˮx��ֵ������ �������������±���
���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
������g�� | m1=5.4 | m2=8.2 | m3=7.4 | m4=7.2 | m5=7.2 |
��Ӧ����____________���������������м��ȣ�����Ҫ���к��ز�����ԭ����__________________��CuSO4��xH2O�е�x=___________������1λС���������ⶨ���xƫ���ܵ�ԭ����____________�����������
a�������¶ȹ��� b����������Ŀ����ϴ� c�����Ⱥ���ڿ�������ȴ




