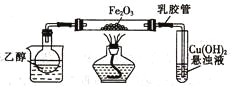
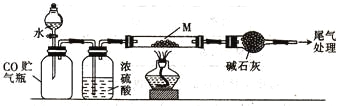
��Ŀ����
����Ŀ���������(NH4ClO4)Ϊ��ɫ���壬���в��ȶ��ԣ���400��ʱ��ʼ�ֽ�����������壬��������������ƽ�����ij��ѧ��ȤС��ͬѧ��������װ�ö�NH4ClO4�ķֽ�������̽����(����װ�����Լ������������ּг�װ����ʡ��)��
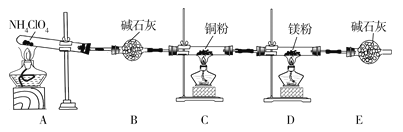
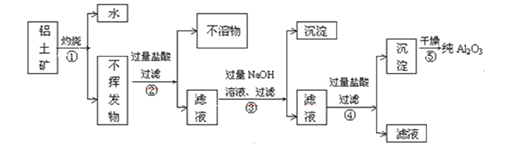
��1����ʵ������з���C��ͭ���ɺ�ɫ��Ϊ��ɫ��˵���ֽ��������__(�ѧʽ)��
��2��ʵ����Ϻ�ȡD��Ӳ�ʲ������еĹ����������Թ��У��μ�����ˮ��������ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬����������Ļ�ѧ����ʽΪ__��
��3��ͨ������ʵ������ķ�����ijͬѧ��Ϊ�����л�Ӧ��H2O��������Cl2����ͬѧ��Ϊ������Cl2���ڵ�������__��
��4��Ϊ��֤��H2O��Cl2�Ĵ��ڣ�ѡ����������װ�ú������ṩ��װ�ý���ʵ�飺
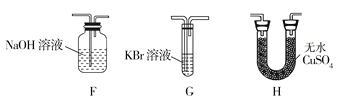
�ٰ������������ң�װ�õ�����˳��ΪA��__��__��__��
��F�з�����Ӧ�����ӷ���ʽΪ__��
��5��ʵ����ۣ�NH4ClO4�ֽ�ʱ�����������������ʣ��������立ֽ�Ļ�ѧ����ʽΪ___��
��6����ʵ�����������E��װ�м�ʯ�ҵ�Ŀ��__��ʵ�������ijͬѧ��ͨ������D��þ�������ı仯�����������淋ķֽ��ʣ�����ɼ�����__(�ƫ��ƫС�������жϡ�)��
���𰸡�O2 Mg3N2+6H2O=3Mg(OH)2��+2NH3�� O2��N2��������������ݻ��ϼ۱仯���ɣ���Ӧ���ڻ�ԭ����Ӷ��жϳ���Ԫ�صĻ��ϼ۽��ͣ���������Cl2 H G F Cl2+2OH-=Cl-+ClO-+H2O 2NH4ClO4![]() N2��+2O2��+Cl2��+4H2O ���տ����е�CO2��ˮ�A�� ƫ��
N2��+2O2��+Cl2��+4H2O ���տ����е�CO2��ˮ�A�� ƫ��
��������
��1��NH4ClO4���ȷֽ���������壬����ʯ�Ҹ������ʹͭ���ɺ�ɫ��Ϊ��ɫ��˵��������CuO�����Էֽ�����к���O2���ʴ�Ϊ��O2��
��2����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ������Ϊ������������ӦΪ��Mg3N2+6H2O=3Mg(OH)2��+2NH3����˵��D�й���ΪMg3N2���ݴ˿��ж�NH4ClO4���ȷֽ��������N2���ɣ��ʴ�Ϊ��Mg3N2+6H2O=3Mg(OH)2��+2NH3����
��3�����ݷ�����֪��NH4ClO4�ֽ�����к���O2��N2��O2��N2���������������������ԭ��Ӧ���ɣ���Ӧ���ڻ�ԭ����Ӷ��жϳ���Ԫ�صĻ��ϼ۽��ͣ����������������ʴ�Ϊ��O2��N2���������������������ԭ��Ӧ���ɣ���Ӧ���ڻ�ԭ����Ӷ��жϳ���Ԫ�صĻ��ϼ۽��ͣ���������Cl2��
��4���ټ���ˮ������������Ӧ������H�е���ˮ����ͭ����ˮ�Ĵ��ڣ������廯�ؼ���������������ˮ��Һ��Ϊ�Ȼ�ɫ��Ϊ�˷�ֹ�����������Ⱦ����������Ҫʹ��β������װ�ã����������������ң�װ�õ�����˳��ΪA��H��G��F���ʴ�Ϊ��H��G��F��
��F�з�����Ӧ������������������Һ���������Ȼ��ơ��������ƺ�ˮ���䷴Ӧ�����ӷ���ʽ��Cl2+2OH=Cl+ClO+H2O���ʴ�Ϊ��Cl2+2OH=Cl+ClO+H2O��
��5��NH4ClO4�ֽ����ɵ�����������������ˮ,��ϵ����غ㡢ԭ���غ���ƽ�ɵã�2NH4ClO4![]() N2��+4H2O+Cl2��+2O2�����ʴ�Ϊ��2NH4ClO4
N2��+4H2O+Cl2��+2O2�����ʴ�Ϊ��2NH4ClO4![]() N2��+4H2O+Cl2��+2O2����
N2��+4H2O+Cl2��+2O2����
��6����ʵ�����������E��װ�м�ʯ�ҵ�Ŀ�������տ����еĶ�����̼��ˮ������ʵ�������ijͬѧ��ͨ������D��þ�������ı仯�����������淋ķֽ��ʣ�þ����װ���е�������������Ӧ����ɲ�������������ɼ�����ƫ�ʴ�Ϊ�����տ����еĶ�����̼��ˮ������ƫ��

����Ŀ��H2S��ʯ�ͻ�����ҵ�㷺���ڵ���Ⱦ�����壬��ͬʱҲ����Ҫ����Դ����Դ����ҵ�Ͽ��Բ�ȡ���ַ�ʽ������
�ɷ�����
��1����֪H2S��ȼ����ΪakJmol-1 ��S��ȼ����ΪbkJmol-1 �������¿���ֱ�������ѳ�H2S�ķ�Ӧ��2H2S(g)��O2(g)=2S(s)��2H2O(l) ��H��______kJmol-1 ��
��2�����������������Ч������Ӧ�������±�����������Ϊ_________��
����� | ������mg��m-3�� | �����¶ȣ��棩 | ����ѹ����MPa�� | �������� |
һ����̼ | ��1.33 | 300��400 | 0��3.0 | �������� |
����̿ | ��1.33 | ���� | 0��3.0 | �������� |
����п | ��1.33 | 350��400 | 0��5.0 | ������ |
�̿� | ��3.99 | 400 | 0��2.0 | ������ |
���ȷֽⷨ����
���ܱ������У�����һ������H2S���壬�����ȷֽⷴӦH2S(g)![]() ���Ʋ�ͬ���¶Ⱥ�ѹǿ����ʵ�飬�����ͼ(a)��
���Ʋ�ͬ���¶Ⱥ�ѹǿ����ʵ�飬�����ͼ(a)��
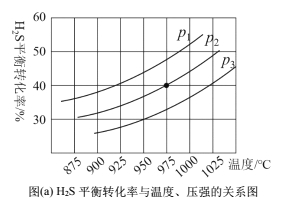
��3��ͼ(a)��ѹǿ��ϵp1��p2��p3�ɴ�С��˳��Ϊ______���÷�ӦΪ____��������������������������Ӧ����Ҫ��һ�����H2S��ƽ��ת���ʣ����˸ı��¶Ⱥ�ѹǿ�⣬�����Բ�ȡ�Ĵ�ʩ��_______��
��4��ѹǿΪp���¶�Ϊ975��ʱ��![]() ��ƽ�ⳣ��K=0.04������ʼŨ��c=______molL��1�������������ټ���1molH2S���壬��ͬ�¶����ٴδﵽƽ��ʱ��K_____0.04������>��1 ��<������=������
��ƽ�ⳣ��K=0.04������ʼŨ��c=______molL��1�������������ټ���1molH2S���壬��ͬ�¶����ٴδﵽƽ��ʱ��K_____0.04������>��1 ��<������=������
��ӵ�ⷨ����
��ӵ�ⷨ��ͨ��FeCl3��Һ���ղ�����H2S���壬����Ӧ����Һͨ�����������ʵ��ѭ��ʹ�ã��÷�������������ͼ(b)��
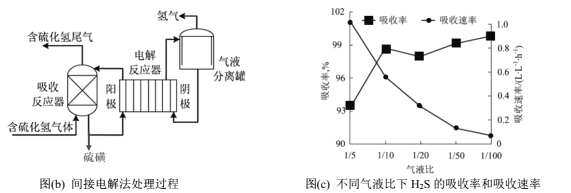
��5����ⷴӦ���ܷ�Ӧ�����ӷ���ʽΪ________��
��6����Һ��Ϊ������Һ������ٱȣ����շ�Ӧ����Һ�����ٹ̶����ⶨ����������ͬʱ���ڲ�ͬ��Һ����H2S�������ʺ��������ʣ������ͼ(c)��ʾ��������Һ�ȼ�С��H2S�������������ͣ��������ʳ��������Ƶ�ԭ��Ϊ____________��