��Ŀ����
[2012���㶫������һģ]��14�֣�I��ij��ȤС��������ɫ������������̽��������Ⱦ��SO2�����ʣ��������ͼʵ��װ�á���ش�
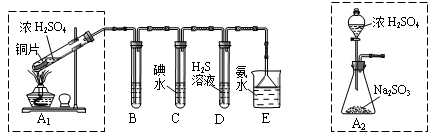
��1��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��
C�з�Ӧ�����ӷ���ʽΪ ��
��2��Ϊ��ʵ����ɫ������Ŀ�꣬ijͬѧ�������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��� ��д���㣩��
II���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ���ش�
��3����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��4����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�

��1��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��
C�з�Ӧ�����ӷ���ʽΪ ��
��2��Ϊ��ʵ����ɫ������Ŀ�꣬ijͬѧ�������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��� ��д���㣩��
II���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ���ش�
��3����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��4����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� |
| ����3�� �� | �� |
��14�֣���1��Ʒ����Һ SO2��I2��2H2O ��SO42-��2 I-��4H+
��2�����ü��ȣ����Լ��Դ��ҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣��κκ�������������֣�
��3��NH3��H2O��SO2��NH4+��HSO3-
��4��
��2�����ü��ȣ����Լ��Դ��ҩƷ�������ȫ�����ڿ��Ʒ�Ӧ���У���Ӧ����֣��κκ�������������֣�
��3��NH3��H2O��SO2��NH4+��HSO3-
��4��
| ʵ����� | Ԥ����������� |
| ����2������1�Σ���������Ʒ����Һ���ٵ������2mol/L���ᣬ�� | ��Ʒ����ɫ���������ݣ���������Һ���д��� SO32-�� |
| ����3�����Թ�ȡ������Һ�������е��������1mol/LBa(OH)2��Һ [�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���� | �����ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ�����������Һ���д��� HSO3-�� |
��1���õ�ˮ����SO2�Ļ�ԭ�ԣ��÷�Ӧ�����ӷ���ʽΪ��SO2��I2��2H2O ��SO42-��2 I-��4H+����H2S����SO2�������ԣ��÷�Ӧ�ķ�Ӧ����ʽΪ��2H2S��SO2��3S����2H2O����Ʒ����Һ����SO2��Ư���ԣ���B����ʢ�Լ�ΪƷ����Һ��
��2����װ��A2��ȡSO2���ŵ��У��ٷ�Ӧ����Ҫ���ȣ��Ƚϰ�ȫ�����÷�Һ©���μӷ�Ӧ��Ũ���ᣬ���ڿ��Ʒ�Ӧ���У��۵μӵ�Ũ�����ܹ���Na2SO3��ַ�Ӧ��
��3��������SO2�백ˮ��Ӧ������������泥���Ӧ�����ӷ���ʽΪ��NH3��H2O��SO2��NH4+��HSO3-��
��4����������Һ���д���SO32-������2�еĹ���ΪBaSO3����ù����е���1�Σ���������Ʒ����Һ���ٵ������2mol/L���ᣬ�������ܽ⣬Ʒ����Һ��ɫ�������ݲ�����
��������Һ���д���HSO3-������2�е���Һ�к���HSO3-��ȡ��������Һ���Թ��У��μӹ�����l mol/LBa(OH)2��Һ���������ɫ��������ȡ��������Һ���Թ��У��μ�1�Σ���������Ʒ����Һ���ٵ���2��3�Σ����������2mol/L���ᣬ��Ʒ����Һ��ɫ�������ݲ�����
��2����װ��A2��ȡSO2���ŵ��У��ٷ�Ӧ����Ҫ���ȣ��Ƚϰ�ȫ�����÷�Һ©���μӷ�Ӧ��Ũ���ᣬ���ڿ��Ʒ�Ӧ���У��۵μӵ�Ũ�����ܹ���Na2SO3��ַ�Ӧ��
��3��������SO2�백ˮ��Ӧ������������泥���Ӧ�����ӷ���ʽΪ��NH3��H2O��SO2��NH4+��HSO3-��
��4����������Һ���д���SO32-������2�еĹ���ΪBaSO3����ù����е���1�Σ���������Ʒ����Һ���ٵ������2mol/L���ᣬ�������ܽ⣬Ʒ����Һ��ɫ�������ݲ�����
��������Һ���д���HSO3-������2�е���Һ�к���HSO3-��ȡ��������Һ���Թ��У��μӹ�����l mol/LBa(OH)2��Һ���������ɫ��������ȡ��������Һ���Թ��У��μ�1�Σ���������Ʒ����Һ���ٵ���2��3�Σ����������2mol/L���ᣬ��Ʒ����Һ��ɫ�������ݲ�����

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
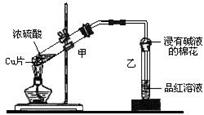
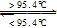 S����б���̣���H=+0.398kJ��mol��1��NAΪ�����ӵ�������������˵���У�������ǣ� ��
S����б���̣���H=+0.398kJ��mol��1��NAΪ�����ӵ�������������˵���У�������ǣ� ��