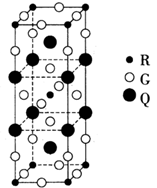
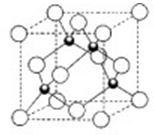
��Ŀ����
��14�֣���1���������Fe(SCN)2+�У��ṩ�չ�����ܹ¶Ե��ӵ�����_________ __��[Cu(H2O)4]2+����Ϊ ���������������е���λ��__________ _______��
��2������VSEPRԤ��HCN�Ŀռ�ṹΪ �Σ�����Cԭ�������γɦҼ����� �� ����������γɦм����� �� �����
��3�����ݼ�ȩ�ķ��ӽṹ�ص��Ʋ��׳ƹ����Ķ��ȼ�ȩ���ӣ�COCl2���ṹʽΪ ������ԭ���ӻ���ʽΪ ���ռ�ṹΪ �Ρ�
��4����Ҫ��д�����ɵ������ڷǽ���Ԫ�ص�ԭ�ӹ���������ԭ��ͨ��sp3�ӻ��γɷ��ӵĻ�ѧʽ����дһ�֣���
�����������____ _���������____ ____��V���_____ ____��
��2������VSEPRԤ��HCN�Ŀռ�ṹΪ �Σ�����Cԭ�������γɦҼ����� �� ����������γɦм����� �� �����
��3�����ݼ�ȩ�ķ��ӽṹ�ص��Ʋ��׳ƹ����Ķ��ȼ�ȩ���ӣ�COCl2���ṹʽΪ ������ԭ���ӻ���ʽΪ ���ռ�ṹΪ �Ρ�
��4����Ҫ��д�����ɵ������ڷǽ���Ԫ�ص�ԭ�ӹ���������ԭ��ͨ��sp3�ӻ��γɷ��ӵĻ�ѧʽ����дһ�֣���
�����������____ _���������____ ____��V���_____ ____��
��14�֣���1��Fe3+(1��)��
��ˮ��ͭ���ӣ�1�֣��� ��1�֣�
��1�֣�
��2��ֱ��(1��)��2(1��)��SP�ӻ�(1��)��2(1��)��δ�ӻ���P���(1��)
��3��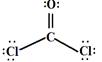 ��1�֣���SP2(1��)��ƽ������(1��)
��1�֣���SP2(1��)��ƽ������(1��)
��4��SiCl4(1��)��PCl3(1��)��SCl2(1��)
��ˮ��ͭ���ӣ�1�֣���
 ��1�֣�
��1�֣���2��ֱ��(1��)��2(1��)��SP�ӻ�(1��)��2(1��)��δ�ӻ���P���(1��)
��3��
 ��1�֣���SP2(1��)��ƽ������(1��)
��1�֣���SP2(1��)��ƽ������(1��)��4��SiCl4(1��)��PCl3(1��)��SCl2(1��)
��1����������ﻯѧʽ��֪��Fe(SCN)2+���ṩ�չ�����ܹ¶Ե��ӵ���Ӧ���������ӣ�[Cu(H2O)4]2+����Ϊ��ˮ��ͭ���ӣ�����ˮ�����壬�������ṩ�չ�������Ը��������е���λ���� ��
��
��2��������HCN������ԭ��̼ԭ��û�й¶Ե��ӣ����Ըû�������ֱ���ͽṹ������Cԭ�������γɦҼ�����2��sp�ӻ��������δ�����ӻ���p������������γɦм���
��3�����ȼ�ȩ���ӣ�COCl2��������̼ԭ��Ҳû�й¶Ե��ӣ�������ƽ�������νṹ�������sp2�ӻ�����ṹʽ��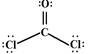 ��
��
��4�����ɵ������ڷǽ���Ԫ�ص�ԭ�ӹ���������ԭ��ͨ��sp3�ӻ��γɷ����У���������������νṹ����Ӧ����SiCl4������������νṹ����Ӧ����PCl3�������ֱ���ͽṹ����Ӧ����SCl2��
 ��
����2��������HCN������ԭ��̼ԭ��û�й¶Ե��ӣ����Ըû�������ֱ���ͽṹ������Cԭ�������γɦҼ�����2��sp�ӻ��������δ�����ӻ���p������������γɦм���
��3�����ȼ�ȩ���ӣ�COCl2��������̼ԭ��Ҳû�й¶Ե��ӣ�������ƽ�������νṹ�������sp2�ӻ�����ṹʽ��
 ��
����4�����ɵ������ڷǽ���Ԫ�ص�ԭ�ӹ���������ԭ��ͨ��sp3�ӻ��γɷ����У���������������νṹ����Ӧ����SiCl4������������νṹ����Ӧ����PCl3�������ֱ���ͽṹ����Ӧ����SCl2��

��ϰ��ϵ�д�
�����Ŀ