��Ŀ����
16��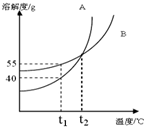 ��ͼ�� A��B ���������ʵ��ܽ�����ߣ���t1��ʱ����25gA��25gB�ֱ�ӵ�����50gˮ�еļס����ձ��У�����ܽ⣬����˵��������ǣ�������
��ͼ�� A��B ���������ʵ��ܽ�����ߣ���t1��ʱ����25gA��25gB�ֱ�ӵ�����50gˮ�еļס����ձ��У�����ܽ⣬����˵��������ǣ�������| A�� | t1��ʱ��A��B�����ʱ�����Һ�����ʵ�����������ͬ | |
| B�� | ��A�����л�������B���ʣ����ý��½ᾧ�����ᴿA | |
| C�� | ��t1��������t2�棬���ձ���A������Һ�����ʵ������������� | |
| D�� | ��t1��������t2�棬���ձ���B������Һ���������� |
���� A�����ݽ���ĺ��������t1��ʱ��A���ܽ��С��B���ܽ�ȣ�
B������ͼ����A��B�����ʵ��ܽ�����¶�Ӱ�첻ͬ��������
C��t1��ʱ��A���ʵ��ܽ��40g����֪���ձ��й���ʣ�࣬��������������������
D������t1��B���ʵ��ܽ�ȿ�֪���ձ��������º����������������䣮
��� �⣺A��t1��ʱ��A���ܽ��С��B���ܽ�ȣ��ʴ��¶�����������Һ������������������ͬ����A����
B����ͼ���֪A���ʵ��ܽ�����¶�Ӱ��ϴ�B���ʵ��ܽ�����¶�Ӱ���С����A�����л�������B���ʣ����ý��½ᾧ�ķ����ᴿA����B��ȷ��
C��t1��ʱ��A���ʵ��ܽ����40g����t1��ʱ����25gA���ʼӵ���ʢ��50gˮ�ļ��ձ��У���һ���ֹ���δ�ܽ⣬��t1�����µ�t2�棬A���ʵ��ܽ������δ�ܽ�Ĺ���ȫ���ܽ⣬���ձ���A������Һ������������������C����
D��t1��ʱ��B���ʵ��ܽ��Լ��57g����t1��ʱ����25gB���ʼӵ���ʢ��50gˮ�����ձ��У�����ȫ���ܽ⣬��t1�����µ�t2�棬���ձ���B������Һ�����ʲ��䣬�����������䣬��D��ȷ��
��ѡAC��
���� �����Կ������ʵ��ܽ�ȼ��ܽ�����ߵ�Ӧ�ã�ѧ���ͼ����ͼ��ͼ�ν���ǽ���Ĺؼ����ڣ�

��1��ֱ���ŷź�SO2���������γ����꣬Σ���������û�ѧ����ʽ��ʾSO2�γ�����������Ĺ���SO2+H2O?H2SO3��2H2SO3+O2=2H2SO4��2SO2+O2$\stackrel{����}{?}$2SO3��SO3+H2O=H2SO4��
��2���ұ���ijС��ͬѧ��õIJ�ͬ��������µ�ij�سǿ�����SO2��ƽ���������������������ٽϴ�ʱSO2ƽ�������ϵ͵�ԭ��
| ������� | ƽ�����٣�m/s�� | ������SO2��ƽ��������mg/L�� |
| ��ǰ | ||
| ��� | ||
| �� | ||
| �� |
�ڷ��ٽϴ�ʱ������Խ��������ɢ�ٶ�Խ�죬������SO2Ũ��ԽС��
��3��ϴ�Ӻ�SO2���������������ʲ�����Ϊϴ�Ӽ�����cd������ĸ��ţ���
a����ʯ�ҡ�����b������c��CaCl2d��NaHSO3
��4��úȼ��ǰ�������������ij���������Ļ���ԭ�����£�FeS2$��_{+O_{2}+H_{2}O}^{������������}$Fe2++SO42-Fe2+
�ٸü����ĵ�һ����Ӧ�����ӷ���ʽΪ2FeS2+7O2+2H2O=4H++2Fe2++4SO42-��
�ڴ���1kg��80% FeS2�Ļ����ڶ�������O2������������Ϊ37.352L������һλС������
��5��ij�о���ѧϰС��Ϊģ��⻯ѧ�������γɣ�������������װ���ܱ������ڵı���Ⱦ������Ʒ���������ʵ�Ũ����ʱ��ı仯��ͼ1��ʾ����ͼ��֪���⻯ѧ������ָO3��ȩ��PAN
����Ⱦ������Ϳ��������γɵ�������

��6������-��ԭ������NOx��ת�����£�NO$��_{��Ӧ��}^{O_{3}}$NO2$��_{��Ӧ��}^{CO��NH_{2}��_{2}}$N2
�ٷ�Ӧ��ΪNO+O3=NO2+O2������11.2L O2�������ʱ��ת�Ƶ��ӵ����ʵ�����1mol��
�ڷ�Ӧ���У���Ӧ�Ļ�ѧ����ʽ��6NO2+4CO��NH2��2=7N2+8H2O+4CO2��
��7�����ð�ˮ���Խ�SO2��NO2���գ�ԭ����ͼ2��ʾ��
NO2�����յ����ӷ���ʽ��2NO2+4HSO3-=N2+4SO42-+4H+����
| A�� | ��ʯ������ͨ�������CO2��OH-+CO2�THCO3- | |
| B�� | amol FeBr2��Һ��ͨ��amol Cl2��2Fe2++2Br-+2Cl2�TBr2+2Fe3++4C1- | |
| C�� | ̼�������Һ�ӵ������У�Ca��HCO3��2+2CH3COOH�TCa2++2CH3COO-+2CO2��+2H2O | |
| D�� | ��50mL 1mol•L-1������Һ�е�������0.1mol•L-1Ba��OH��2��Һ��Al3++2SO42-+2Ba2++4OH-�TAlO2-+2BaSO4��+2H2O |
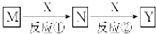
| A�� | M��Na X��O2 | B�� | M��HNO3 X��Fe | C�� | M��NH3 X��O2 | D�� | M��Al X��NaOH |
| A�� | H+��Ħ��������1g | B�� | H+��Ħ��������1g/mol | ||
| C�� | H+��Ħ��������1 | D�� | H+��Ħ��������1mol |
 ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ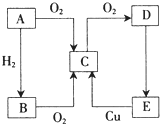 A��B��C��D��E������������ͼ��ʾ��ת����ϵ��������A��һ�ֵ���ɫ�Ĺ��壮
A��B��C��D��E������������ͼ��ʾ��ת����ϵ��������A��һ�ֵ���ɫ�Ĺ��壮