��Ŀ����
4�� ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ�밴Ҫ��ش���������
��1��SO2������Fe3+��Ӧ�����ӷ���ʽ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+
��2������ʵ�鷽����������ʵ������ȡ����SO2����B��
A��Na2SO3��Һ��HNO3������B��Na2SO3������Ũ���� ��C���������ڴ�����ȼ��
��3����Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���Ȼ�������һϵ�в�����û���õ��������У�����ţ�B��
A�������� B��ʯ���� C��©�� D��������
��4��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�������KMnO4��Һ����ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ����죬�ټ������Ƶ���ˮ����Һ��죻
�����ۣ�����������Һ�м����������ữ��BaCl2��Һ��������ɫ������
�����������ķ����Ƿ����٣�ԭ������ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
��5���ܱ���I-�Ļ�ԭ������SO2��������B����ɫ��Һ��ɫ��
���� ��1������װ��A��Ӧ�����ӷ���ʽSO2+2Fe3++2H2O�T2Fe2++SO42-+4H+���н�𣻸��ݻ��ϼ۱仯�жϷ�Ӧ��SO2��Fe3+�����ʵ���֮�ȣ�
��2��ʵ������ȡ����Ҫ���Dz������㡢���ơ����ܺ����ʣ�
��3����A��������Һ��ȡ����Ϊ�̷�����Һ�еõ��̷���ʵ�����������Ũ������ȴ�ᾧ������ϴ�ӣ����ﲽ��õ��������õ�������ʯ�������������������õ�©�����ձ������������ݴ˽��н��
��4�������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ��
��5������������ʹ���е�ĵ�����Һ��ɫ��˵��������������ԭ��Ӧ�����ݻ�ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�Է�����
��� �⣺��1�����������������ӷ�Ӧ������������Ӻ��������ӣ���Ӧ�����ӷ���ʽΪ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
�ʴ�Ϊ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��2��A���������ǿ�����ԣ��ܹ����������������������ƣ����õ������������壬��A����
B��Ũ���������ǿ���ԣ���Ũ����ӷ�������������Һ��Ũ�����ܹ���Ӧ���ɶ����������壬��B��ȷ��
C���������ڴ�����ȼ�գ������������ƣ�������ô����Ķ�������C����
�ʴ�Ϊ��B��
��3����Һ�еõ��̷���ʵ�����Ϊ������Ũ������ȴ�ᾧ������ϴ�ӡ����
��������ʹ�������У�A��������D����������������ʹ������Ϊ��C��©����D��������������û��ʹ�õ����ǣ�B��ʯ������
�ʴ�Ϊ�����ˣ�B��
��4�����������л�ԭ�ԣ����������ǿ�����ԣ������������������ط���������ԭ��Ӧʹ���������Һ��ɫ��Fe2+Ҳʹ���������Һ��ɫ�����Է����ٲ�������
�ʴ�Ϊ�������٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ��
��5��I2+SO2+2H2O=2HI+H2SO4����������ʹ���е�ĵ�����Һ��ɫ��˵��������������ԭ��Ӧ�����������������������ǻ�ԭ������ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ�
�ʴ�Ϊ��B����ɫ��Һ��ɫ��
���� ���⿼������������ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ������漰������ԭ��Ӧ�ļ��㡢�����Ի�ԭ��ǿ���Ƚϡ�����ʵ�鷽������������۵�֪ʶ������ѧ�����������ͼ��������Ŀ��飬��ȷ����Ũ��������ʡ���������ļ��鷽����֪ʶΪ�����ؼ���

 ����������������ϵ�д�
����������������ϵ�д�| A�� | ���³�ѹ��18gˮ�к��е�ԭ������Ϊ3NA�� | |
| B�� | ��������ΪNA��NO2��CO2��������к��е���ԭ����Ϊ2NA | |
| C�� | ��״����2.24LH2O������������ΪNA | |
| D�� | ���³�ѹ�£�1.7gH2O2�к��еĵ�����Ϊ0.9NA |
| A�� | ��Һ��� | B�� | ��Һ���� | C�� | �ȱ�����ɫ | D�� | ����ɫ |
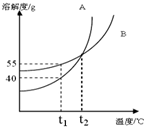 ��ͼ�� A��B ���������ʵ��ܽ�����ߣ���t1��ʱ����25gA��25gB�ֱ�ӵ�����50gˮ�еļס����ձ��У�����ܽ⣬����˵��������ǣ�������
��ͼ�� A��B ���������ʵ��ܽ�����ߣ���t1��ʱ����25gA��25gB�ֱ�ӵ�����50gˮ�еļס����ձ��У�����ܽ⣬����˵��������ǣ�������| A�� | t1��ʱ��A��B�����ʱ�����Һ�����ʵ�����������ͬ | |
| B�� | ��A�����л�������B���ʣ����ý��½ᾧ�����ᴿA | |
| C�� | ��t1��������t2�棬���ձ���A������Һ�����ʵ������������� | |
| D�� | ��t1��������t2�棬���ձ���B������Һ���������� |
| A�� | 1.7g H2O2�к��еĵ�����Ϊ0.9 NA | |
| B�� | �����ʵ�����N2��CO������������ΪNA | |
| C�� | 1mol Na2O2 �����к���������Ϊ4 NA | |
| D�� | ��״���£�2.24L��������������Ϊ0.1 NA |
