��Ŀ����
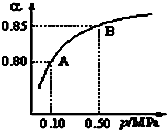
11������ͼ��ʾ���ձ��ж�ʢ��ϡ���ᣮ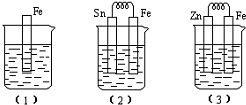
��1���з�Ӧ�����ӷ���ʽΪFe+2H+=Fe2++H2����
��2���еĵ缫��ӦʽΪ��������Fe-2e=Fe2+��������2H++2e=H2��
Sn��������Һ��pH����������С�䣩������Ӧ��������1molH2����ʱ��������ת�Ƶĵ�����ĿΪ2NA����NA��ʾ����
��3���б���ʴ�Ľ�����Zn����缫��ӦʽΪZn-2e�TZn2+��
�����Ƚϣ�1������2������3���д�������ʴ�������ɿ쵽����˳���ǣ�2������1������3����
���� ��1��������ϡ���ᷢ���û���Ӧ��
��2��������ʧ������������Sn����������������ʧ���ӷ���������Ӧ�������������ӷŵ磻
��3����װ���У�п��ʧ������������Fe���������������Ľ����ױ���ʴ��
����������ʴ����˳���ǣ���ԭ��ظ�������ѧ��ʴ����ԭ��������ĵ缫��
��� �⣺��1��������ϡ���ᷢ���û���Ӧ�����������Ӻ��������缫��ӦʽΪFe+2H+=Fe2++H2�����ʴ�Ϊ��Fe+2H+=Fe2++H2����
��2����װ����Fe��ʧ������������Sn�������������缫��ӦΪFe-2e=Fe2+�������缫��ӦΪ2H++2e=H2������Һ�������ӷŵ絼����Һ������Ũ�Ƚ��ͣ�����Һ��pH������Ӧ��������1molH2����ʱ��������ת�Ƶĵ�����ĿΪ2NA���ʴ�Ϊ��Fe-2e=Fe2+��2H++2e=H2��������2NA��
��3����װ���У�п��ʧ������������Fe����������ԭ��ظ����Ľ����ױ���ʴ�����Ա���ʴ�Ľ�����Zn���缫��ӦΪZn-2e�TZn2+���ʴ�Ϊ��Zn��Zn-2e�TZn2+��
����������ʴ����˳���ǣ���ԭ��ظ�������ѧ��ʴ����ԭ��������ĵ缫������������ʴ����˳���ǣ�2������1������3����
�ʴ�Ϊ����2������1������3����
���� ���⿼����ԭ���ԭ��������ʧ�������׳̶�ȷ����������֪��������ʴ����˳����Ŀ�ѶȲ���

��CH3Cl ��CH2Cl2 ��CHCl3 ��CCl4��
| A�� | ֻ�Т� | B�� | �٢ڢۢܵĻ���� | C�� | ֻ�Т� | D�� | �ٺ͢۵Ļ���� |
| A�� | ��ˮ$\stackrel{NaOH}{��}$Mg��OH��2$\stackrel{���}{��}$Mg | |
| B�� | ��ˮ$\stackrel{HCl}{��}$ MgCl2��Һ��MgCl2����$\stackrel{���}{��}$Mg | |
| C�� | ��ˮ$\stackrel{ʯ����}{��}$Mg��OH��2$\stackrel{����}{��}$ MgO$\stackrel{���}{��}$ Mg | |
| D�� | ��ˮ$\stackrel{ʯ����}{��}$Mg��OH��2$\stackrel{HCl}{��}$MgCl2��Һ��MgCl2����$\stackrel{���}{��}$Mg |
| A�� | H2S | B�� | CO2 | C�� | SO2 | D�� | H2 |
| A�� | Ksp��AgI����Ksp��AgCl�� | |
| B�� | �ӹ���KI��Һ��ַ�Ӧ����Һ��Ag+��I-��Ũ��֮������Ksp��AgI�� | |
| C�� | �ӹ���KI��Һ��ַ�Ӧ����Һ��Ag+��Cl-��Ũ��֮������Ksp��AgCl�� | |
| D�� | ���Ͻ��۶�����ȷ |
| A�� | pHֵ��Ϊ12���ռ���Һ������������Һ�����ʵ���Ũ��֮�� | |
| B�� | ����Һ��c��K+����c��S2-��֮�� | |
| C�� | ��ͬ�¶��£�0.2mol•L-1�Ĵ�����Һ��0.1mol•L-1�Ĵ�����Һ�е�c��H+��֮�� | |
| D�� | 10mL 0.5mol•L-1��������Һ��5mL 0.5mol•L-1�Ĵ�����Һ�е�n��H+��֮�� |
 ij����װ����ͼ��ʾ��
ij����װ����ͼ��ʾ��