��Ŀ����
19������ȼ�ϵ�أ�Microbial Fuel Cell��MFC����һ���������ォ�л����еĻ�ѧ��ֱ��ת���ɵ��ܵ�װ�ã����������л���ˮ��������ͼ����������ȼ�ϵ�ش�������ȩ��ˮ��װ�ã�����3�����ӽ���Ĥ�������й�˵������ȷ���ǣ���������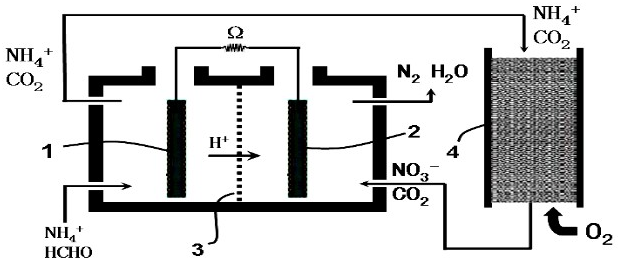
| A�� | ���������ķ�ӦΪ��HCHO-4e-+H2O�TCO2+4H+ | |
| B�� | �������ڵ��������豣���������� | |
| C�� | NH4+ͨ��ѭ���������ձ�ת����N2 | |
| D�� | O2����������Ӧ��������ԭ��Ӧ |
���� ��H+�Ķ����ƶ���֪����ȩΪ����������������Ӧ����Ӧ�ĵ缫��Ӧʽ��HCHO-4e-+H2O=CO2+4H+��NH4+������������������NO3-������NO3-����ԭ����N2���Դ˽����⣮
��� �⣺A����ȩΪ����������������Ӧ����Ӧ�ĵ缫��Ӧʽ��HCHO-4e-+H2O=CO2+4H+����A��ȷ��
B��Ϊ��ֹ������ȩ�������������γ�ԭ��ط�Ӧ�������ڵ��������豣��������������B��ȷ��
C��NH4+������������������NO3-������NO3-����ԭ����N2����C��ȷ��
D������NO3-����ԭ����N2�������������μ�������Ӧ����D����
��ѡD��
���� ���⿼����ԭ���ԭ����Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬��ȷԭ����������ϵ�ʧ���ӡ��������������Һ�����������ƶ����ɽ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
19������˵����ȷ���ǣ�������
| A�� | ��������ά�ػ�Ϊͬ���칹�� | |
| B�� | ��ϩ�ܺ���ˮ�����Ը��������Һ�����ӳɷ�Ӧʹ֮��ɫ | |
| C�� | ���͡����ͺ�ֲ���Ͷ���̼�⻯�����ȫȼ��ֻ����CO2��H2O | |
| D�� | ���ۡ���֬�������ʶ���ˮ�⣬��ˮ����ﲻͬ |
20������Һ���ܴ�������������ǣ�������
| A�� | K+��CH3COOH��CO32-��NO3- | B�� | Fe3+��Na+��SCN-��Cl- | ||
| C�� | Ba2+��Na+��OH-��HCO3- | D�� | H+��K+��Fe3+��NO3- |
7���谢��ӵ�����ΪNA������������ȷ���ǣ�������
| A�� | 1Ħ��H2O����������Ϊ12NA | |
| B�� | 2����������ԭ����ΪNA | |
| C�� | 0.5Ħ�����������������ᷴӦת�Ƶ�����Ϊ1.5NA | |
| D�� | ��״���£�1��ˮ����������Ϊ$\frac{1}{22.4}$ NA |
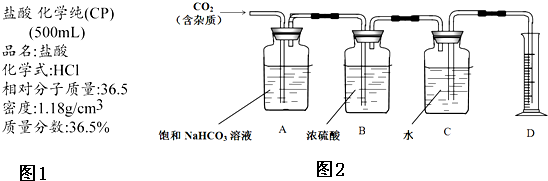
 ��1���������������ɺ����ʿɽ�����ͼ���ࣺ��ͼ��ʾ�ķ��������B������ţ�
��1���������������ɺ����ʿɽ�����ͼ���ࣺ��ͼ��ʾ�ķ��������B������ţ� �۰��ס�����������2��2-�������顡����ˮ ��
�۰��ס�����������2��2-�������顡����ˮ ��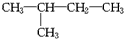 ��CH3��CH2��3CH3����${\;}_{17}^{35}$Cl�������⑪${\;}_{17}^{37}$Cl⑫
��CH3��CH2��3CH3����${\;}_{17}^{35}$Cl�������⑪${\;}_{17}^{37}$Cl⑫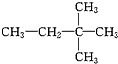 ⑬CH2=CH2⑭CH2=CH-CH=CH2
⑬CH2=CH2⑭CH2=CH-CH=CH2 ��ȡ����Ӧ��
��ȡ����Ӧ�� ��ȡ����Ӧ��
��ȡ����Ӧ��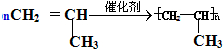 ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��