题目内容
【题目】A、B、C、D、E代表前四周期原子序数依次增大的五种元素。A、D同主族且有两种常见化合物DA2和DA3;工业上电解熔融C2A3制取单质C;B、E除最外层均只有2个电子外,其余各层全充满。回答下列问题:
(1)B、C中第一电离能较大的是______________(填元素符号),基态D原子价电子的轨道表达式为______________。
(2)DA2分子的VSEPR模型是______________。H2A比H2D熔沸点高得多的原因是______________。

(3)实验测得C与氯元素形成化合物的实际组成为C2Cl6,其球棍模型如图所示。已知C2Cl6 在加热时易升华,与过量的NaOH溶液反应可生成Na[C(OH)4]。
①C2Cl6属于______________晶体(填晶体类型),其中C原子的杂化轨道类型为______________杂化。
②[C(OH)4]-中存在的化学键有______________。
(4)工业上制备B的单质是电解熔融B的氯化物,而不是电解BA,原因是__________。

(5)B、C的氟化物晶格能分别是2957kJ·mol-1、5492kJ·mol-1,二者相差很大的原因是______________。
(6)D与E所形成化合物晶体的晶胞如右图所示。
①在该晶胞中,E的配位数为____________。
②原子坐标参数可表示晶胞内部各原子的相对位置。右图晶胞中,原子坐标参数a为(0,0,0);b为(![]() ,0,
,0,![]() );c为(
);c为(![]() ,
,![]() ,0)。则d原子(面心上)的坐标参数为______________。
,0)。则d原子(面心上)的坐标参数为______________。
③已知该晶胞的密度为ρg/cm3,则其中两个D原子之间的距离为______________pm(列出计算式即可)。
【答案】 Mg ![]() 平面三角形 H2O分子间存在氢键 分子 sp3 极性共价键、配位键(或共价键、配位键) 熔融MgCl2能导电,可电解;MgO熔沸点高,电解熔融MgO能耗大 Al3+比Mg2+电荷高、半径小,AlF3的晶格能比MgF2大得多 4 (1,
平面三角形 H2O分子间存在氢键 分子 sp3 极性共价键、配位键(或共价键、配位键) 熔融MgCl2能导电,可电解;MgO熔沸点高,电解熔融MgO能耗大 Al3+比Mg2+电荷高、半径小,AlF3的晶格能比MgF2大得多 4 (1,![]() ,
,![]() )
) ![]() ×
×![]() ×1010
×1010
【解析】A、B、C、D、E代表前四周期原子序数依次增大的五种元素。A、D同主族且有两种常见化合物DA2和DA3,A是O,D是S。工业上电解熔融C2A3制取单质C,C是Al;B、E除最外层均只有2个电子外,其余各层全充满,所以B是Mg,E是Zn。则(1)镁的3s轨道电子处于全充满状态,稳定性强,则B、C中第一电离能较大的是Mg,基态S原子价电子的轨道表达式为![]() 。(2)SO2分子中S原子的价层电子对数=2+(6-2×2)/2=3,所以VSEPR模型是平面三角形。由于H2O分子间存在氢键,所以H2O比H2S熔沸点高得多。(3)①受热易升华,说明Al2Cl6属于分子晶体,分子中Al形成4个共价键,则Al原子的杂化轨道类型为sp3杂化。②[Al(OH)4]-中存在的化学键有配位键、共价键。(4)由于熔融MgCl2能导电,可电解;MgO熔沸点高,电解熔融MgO能耗大,所以工业上制备Mg的单质是电解熔融Mg的氯化物,而不是电解氧化镁。(5)B、C的氟化物均是离子晶体,由于Al3+比Mg2+电荷高、半径小,因此AlF3的晶格能比MgF2大得多。(6)①根据晶胞结构可判断该晶胞中,Zn的配位数为4。②根据晶胞结构并参照a、b、c的原子坐标参数可知d原子(面心上)的坐标参数为(1,
。(2)SO2分子中S原子的价层电子对数=2+(6-2×2)/2=3,所以VSEPR模型是平面三角形。由于H2O分子间存在氢键,所以H2O比H2S熔沸点高得多。(3)①受热易升华,说明Al2Cl6属于分子晶体,分子中Al形成4个共价键,则Al原子的杂化轨道类型为sp3杂化。②[Al(OH)4]-中存在的化学键有配位键、共价键。(4)由于熔融MgCl2能导电,可电解;MgO熔沸点高,电解熔融MgO能耗大,所以工业上制备Mg的单质是电解熔融Mg的氯化物,而不是电解氧化镁。(5)B、C的氟化物均是离子晶体,由于Al3+比Mg2+电荷高、半径小,因此AlF3的晶格能比MgF2大得多。(6)①根据晶胞结构可判断该晶胞中,Zn的配位数为4。②根据晶胞结构并参照a、b、c的原子坐标参数可知d原子(面心上)的坐标参数为(1,![]() ,
,![]() )。③根据晶胞结构可知Zn原子个数=8×1/8+6×1/2=4,S原子个数=4,已知该晶胞的密度为ρg/cm3,则晶胞的边长=
)。③根据晶胞结构可知Zn原子个数=8×1/8+6×1/2=4,S原子个数=4,已知该晶胞的密度为ρg/cm3,则晶胞的边长=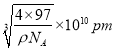 ,其中两个S原子之间的距离为面对角线的一半,则为
,其中两个S原子之间的距离为面对角线的一半,则为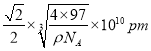 。
。

 浙江名校名师金卷系列答案
浙江名校名师金卷系列答案