��Ŀ����
4����һ����������ͨ����������Ũ��Һ�У���������ʱ�����Һ���γ�NaCl��NaClO��NaClO3�Ĺ�����ϵ�������жϲ���ȷ���ǣ�������| A�� | �μӷ�Ӧ����NaOH�����������ʵ���֮��һ��Ϊ2��1 | |
| B�� | ��Ӧ��ϵ��n��NaCl����n��NaClO����n��NaC1O3�������ʵ���֮�ȣ�����Ϊ11��1��2 | |
| C�� | ����Ӧ��amol�����μӷ�Ӧ����amol��ת�Ƶ�������$\frac{5a}{3}$mol | |
| D�� | ��Ӧ��NaC1O��NaClO3Ϊ������������ʵ���֮��һ��Ϊl��1 |
���� ������Ӧ�У�Cl2+2NaOH=NaCl+NaClO+H2O��3Cl2+6NaOH=5NaCl+NaClO3+3H2O��
A�����������Ӻ���ԭ�������غ�����μӷ�Ӧ���������ƺ����������ʵ���֮�ȣ�
B������������ԭ��Ӧ�е�ʧ��������жϣ�
C�����ݼ�ֵ���������غ�����ת�Ƶ��ӵķ�Χ��
D������ȱ�ٴ������ơ������Ƶ�������������ߵ����ʵ���֮�ȣ�
��� �⣺A������NaCl��NaClO��NaClO3��֪�������Ӻ���ԭ�ӵ����ʵ���֮��Ϊ1��1����μӷ�Ӧ����NaOH�����������ʵ���֮��һ��Ϊ1��$\frac{1}{2}$=2��1����A��ȷ��
B����n��NaCl��=11mol��n��NaClO��=1mol��n��NaClO3��=2mol������NaCl��õĵ���Ϊ��11mol��1=11mol������NaClO��NaClO3ʧȥ�ĵ���Ϊ1mol��1+2mol��5=11mol����ʧ������ȣ��ʷ�Ӧ��ϵ��n��NaCl����n��NaClO����n��NaC1O3�������ʵ���֮�ȣ�����Ϊ11��1��2����B��ȷ��
C����amol�������뷴Ӧ��������Ӧ�У�Cl2+2NaOH=NaCl+NaClO+H2O��3Cl2+6NaOH=5NaCl+NaClO3+3H2O������������ֻ��NaClO��ת�Ƶ��������٣�Ϊamol��2��$\frac{1}{2}$��1=amol����������ֻ��NaClO3��ת�Ƶ�������࣬Ϊamol��2��$\frac{5}{6}$��1=$\frac{5a}{3}$mol�����Է�Ӧת�Ƶ���Ϊ��amol��ת�Ƶ�������$\frac{5a}{3}$mol����C��ȷ��
D�����ݻ��ϼ۱仯��֪����Ӧ��NaC1O��NaClO3Ϊ�������������ȱ�����ݣ���������ߵ����ʵ���֮�ȣ���D����
��ѡD��
���� ���⿼����������ԭ��Ӧ���йؼ��㣬��Ŀ�Ѷ��еȣ�����ԭ���غ㼰ת�Ƶ����غ��ǽⱾ��ؼ�������ϼ��������������������ѧ���ķ�����������ѧ����������

| A�� | ǿ������ | B�� | ���� | C�� | ��ԭ�� | D�� | �ȶ��� |
| A�� | ���³�ѹ�£�����Ϊ32gO2���е�ԭ����Ϊ2NA | |
| B�� | 2L0.1mol/LNaCl��Һ�к���NaCl������Ϊ0.2NA | |
| C�� | 78gNa2O2������CO2��ȫ��Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| D�� | ��25�棬101Pa�����£�11.2L����������ԭ����ΪNA |
| A�� | ������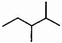 ��ϵͳ������������Ϊ2��3һ�������� ��ϵͳ������������Ϊ2��3һ�������� | |
| B�� | ���ۺ���ά�صĻ�ѧʽ��Ϊ��C6H10O5��n������Ϊͬ���칹�� | |
| C�� | �������ľ���ϩ�����顢��ϩ�ֱ���ȼ�գ����������������μ��� | |
| D�� | ͨ���þƾ���������ԭ���Ǿƾ�ʹϸ���еĵ����ʱ��Զ�ʧȥ�������� |
| A�� | 25��ʱ��1L pH=13��Ba��OH��2��Һ�к���OHһ����ĿΪ0.2NA | |
| B�� | ȡ50mL 14.0moI��L-lŨ������������ͭƬ��Ӧ������������ӵ���ĿΪ0.35NA | |
| C�� | ��״���£�2.24L���������к��еĵ�������Ϊ3.2NA | |
| D�� | 28gN2��28g C18O�к��е���������Ϊ14 |
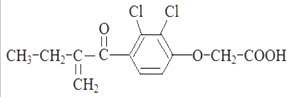
| A�� | ����ʽ��C13H14O4Cl2 | |
| B�� | һ���������ܷ�����ȥ��Ӧ | |
| C�� | -���������ܷ����Ӿ۷�Ӧ | |
| D�� | 1mol�������������5molH2�����ӳɷ�Ӧ |
| A�� | ��Ԫ�ط�����ȷ���л�����������ʱ����������Ʒ��С�������ٶȿ���ŵ� | |
| B�� | ���ڷ����Զ���ȷ���л������Ƿ���C��H��O��Ԫ�� | |
| C�� | �ⶨ�л����������Ԫ�صķ������к˴Ź�������ȷ��� | |
| D�� | ���Ѻ��Ҵ���ͬ���칹�壬�����ں˴Ź������г��ֵ�������ֱ���1����2�� |
| A�� | ij��ɫ��Һ�У�NH4+��Na+��Cl-��MnO4- | |
| B�� | ��������ˮ�������c��H+��=1��10-13mol•L-1����Һ�У�Na+��K+��SO32-��CO32- | |
| C�� | ��c��H+��=1��10-13mol•L-1����Һ�У�NH4+��Al3+��SO42-��NO3- | |
| D�� | ��pH=1����Һ�У�K+��Mg2+ SiO32- SO42- |
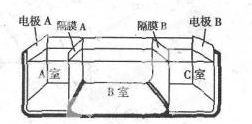 ˮ��һ����Ҫ����Ȼ��Դ���������������治��ȱ�ٵ����ʣ���ش��������⣺
ˮ��һ����Ҫ����Ȼ��Դ���������������治��ȱ�ٵ����ʣ���ش��������⣺